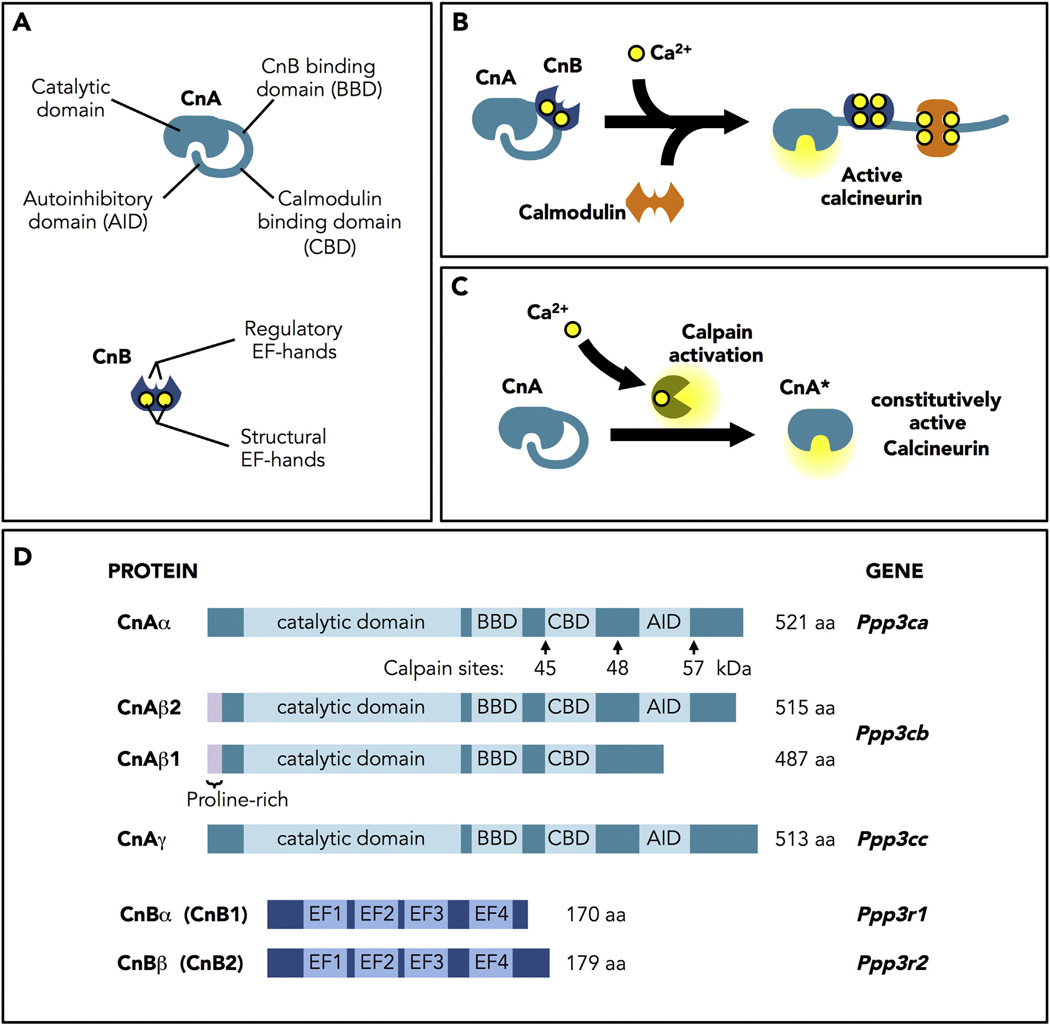
Fig. 1.
Calcineurin structure, regulation and activation. A. Structure of the catalytic (CnA) and regulatory (CnB) subunits of calcineurin. CnA contains an N-terminal catalytic domain, a CnB binding domain, a calmodulin binding domain, and a C-terminal autoinhibitory domain (AID). CnB has four EF-hand Ca2+-binding sites: two structural sites that bind Ca2+ with high affinity in the ηM range, that are always occupied, and two regulatory sites that bind in the µM range. B. Model of calcineurin activation. Binding of Ca2+ to the regulatory sites initiates a series of conformational changes that allow binding of a calmodulin/Ca2+ complex and a change in the orientation of the AID to expose the active site. C. Constitutively active calcineurin. Truncation of CnA to remove the AID yields a constitutively active phosphatase (CnA*) that no longer responds to Ca2+/calmodulin. D. Calcineurin proteins and genes. In mammals, three genes encode CnA (α, β, and γ). Only CnAα (PPP3CA) and CnAβ (PPP3CB) are expressed in the heart. Of the two genes encoding the CnB regulatory subunit, only CnBα (PPP3R1) is expressed in the heart.