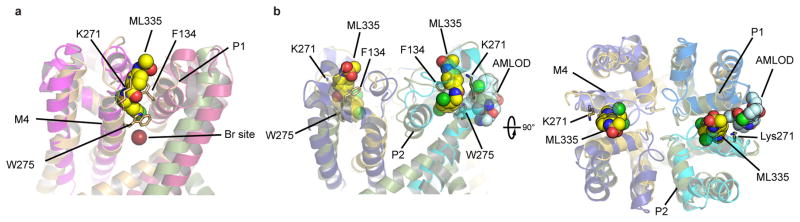
Extended Data Figure 3. Comparison of K2P modulator and VGIC antagonist sites.
a, Superposition of the K2P2.1(TREK-1):ML335 complex (smudge and light orange) with the BacNaV ‘pore-only’ NaVMs structure18 (magenta and warm pink). Bromine site (Br) from labeled sodium channel antagonists is shown as a firebrick sphere. b, Superposition of the pore domains of the K2P2.1(TREK-1):ML335 complex (smudge and light orange) with the pore domain of the BacNaV CaVAb (5KMD) bound to the inhibitor amlodipine (AMLOD)19, a site normally occupied by lipid19,37. Select residues of the K2P modulator pocket are shown as sticks and are labeled. CaVAb subunits are colored cyan, marine, slate, and dark blue. ML335 (yellow) and amlodipine (cyan) are shown in space filling representation.