Figure 3.
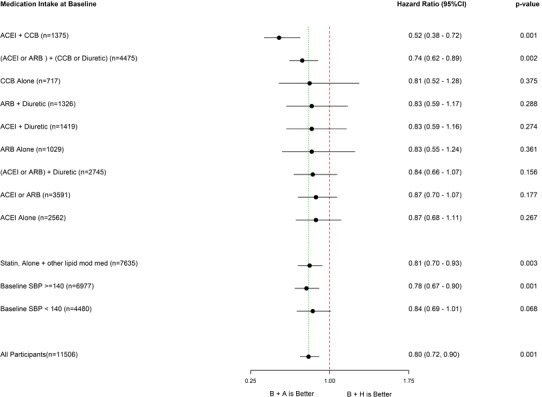
Composite trial outcomes among additional secondary subgroups. Forest plot representing the adjusted hazard ratios±95% confidence intervals by Cox proportional hazard model in favor of benazepril (B)+amlodipine (A) therapy by secondary subgroups. In each antihypertensive medication subgroup, participants were taking the specific medication(s) listed without overlap between unique subgroups. Patients could not be included in >1 subgroup. For example, patients in the calcium channel blocker (CCB) alone, ACEI (angiotensin‐converting enzyme inhibitor) alone, ACEI+CCB, and ACEI+diuretic subgroups were all different individuals. The exception is that there were overlaps in patients between subgroups containing the term “or” in the definition. For example, patients could be in both the ACEI+diuretic group and the ACEI or angiotensin receptor blocker [ARB]+diuretic subgroup. Patients using other antihypertensive agents not listed in the figure (eg, β or α blockers) were not excluded from these subgroups. Other subgroups listed include background lipid‐lowering therapy and systolic blood pressure (SBP) control status on trial randomization. CI indicates confidence interval; H, hydrochlorothiazide; HMG‐CoA, 3‐hydroxy‐3‐methylglutaryl coenzyme‐A reductase inhibitor (statin); and mod, modifying.
