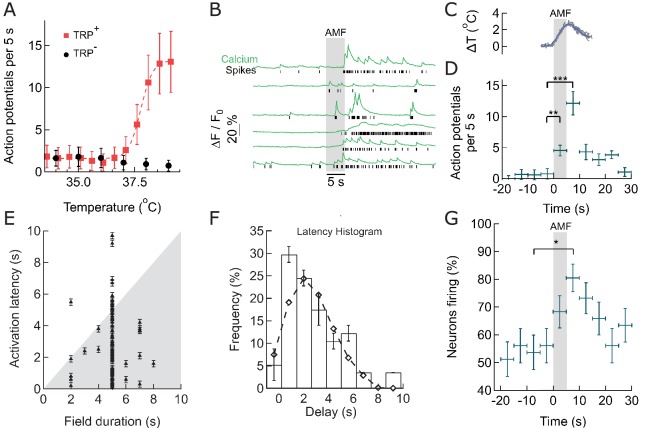
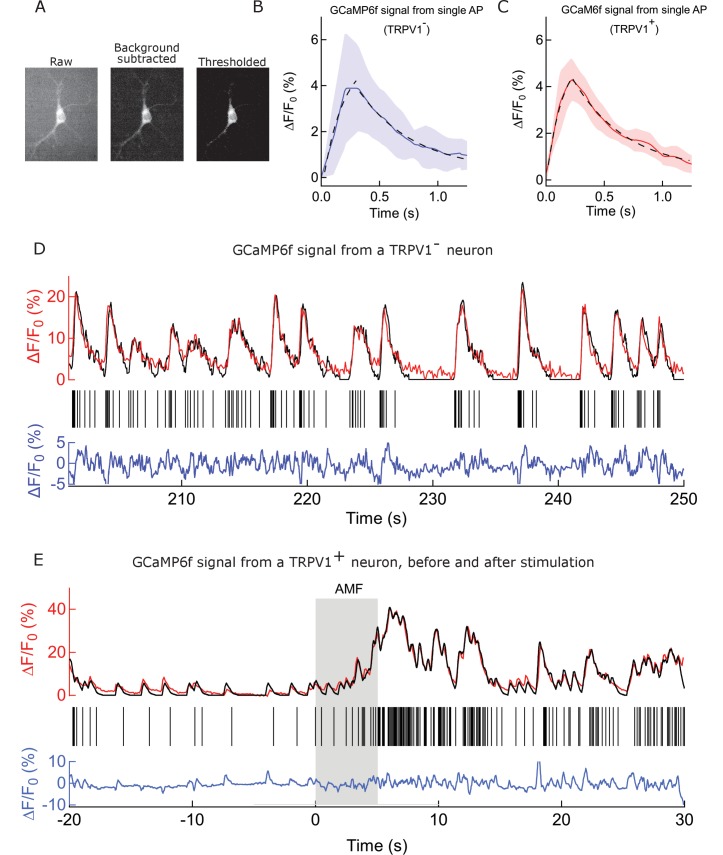
Figure 3. Within seconds of AMF application, membrane-targeted MNP stimulate magnetothermally TPRV1+ neurons in culture.
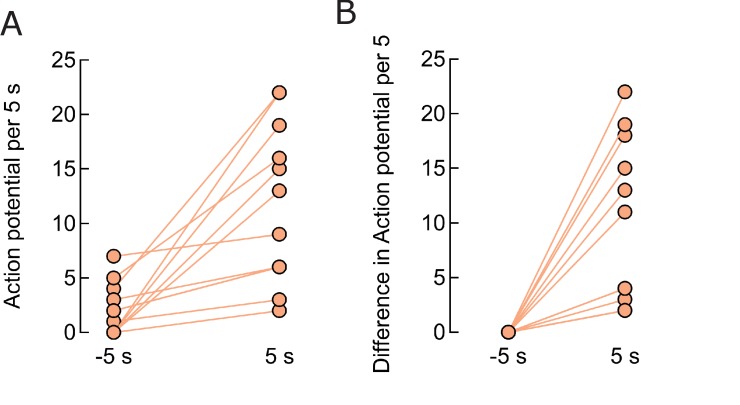
(A) Rate of Action Potential firing as a function of bath temperature, recorded from GCaMP6f transients observed in TRPV1 expressing hippocampal neurons (red) and wild-type neurons (control, black) when perfused with pre-heated buffer. The Ca2+ transients are modeled by a spike train (see Figure 3—figure supplement 1). The data points are fitted with Hill equation, giving a midpoint of 37.7 ± 0.06°C, which corresponds to half maximal firing rate (dashed curve). (B) GCaMP6f fluorescence intensity changes (green, Ca2+) in different TPRV1 +neurons decorated with MNP (5 s field, 22.4 kA/m at 412.5 kHz, in gray). Calculated spike events (black) are indicated under each Ca2+ trace (see Figure 3—figure supplement 2). (C) Change of cell surface temperature as measured by DyLight550 fluorescence (average of three experiments). (D) GCaMP6f signal recorded from nanoparticle-coated TRPV1 +neurons binned in 5 s intervals, indicated by x error bar (mean ±s.e.m, 13 neurons). The spiking frequency increased from 1.8 ± 0.6 per 5 s, before AMF (field 0 to 5 s), to 4.5 ± 1.2 per 5 s, during the AMF (n = 13, **p=0.0028, unpaired T-test), and 12.1 ± 2.0 per 5 s immediately following the AMF (n = 13, ***p=0.0002, unpaired T-test, 95% confidence intervals [1.5,2.23] and [10.8,13.3]) (Supplement 2). (E) Plot of activation latency, time interval between onset of field stimulation and first AP detect versus field duration. All data points lying on the gray background indicate that the first spike was detected while the field was still on (87% of events, n = 79, six cultures). Error bars indicate the temporal measurement uncertainty. (F) Percentage of neurons firing their first AP after field onset in the time interval indicated (subset of 41 cells from A which were stimulated for 5 s). The histogram was fitted (no weighting) with a Poisson curve (λ = 2.18 ± 0.17 s). Error bars are obtained as difference in population between bins shifted to left and right, following temporal uncertainty as indicated in (E). (G) Percentage of active neurons in each time interval (Alternating magnetic field applied 0–5 s). Error bars: x indicate 5 s time bin, y as in (F). A five-second AMF application increased the active population from 53.7 1.6% to 80.5 5.1% of neurons, *p=0.032, unpaired T-test, (n = 41, same as in (F)).