Abstract
Objective
The prognostic value of a high platelet-lymphocyte ratio (PLR) has been reported in patients with non-ST elevated myocardial infarction (NSTEMI) and different oncologic disorders. We aimed to evaluate the predictive value of the PLR for left ventricular systolic dysfunction (LVSD) in patients with non-ST elevated acute coronary syndrome (NST-ACS).
Methods
A total of 220 patients with NST-ACS were included in the study. The study population was divided into tertiles based on admission PLR values. High (n=73) and low PLR (n=147) groups were defined as patients having values in the third tertile (>135.6) and lower 2 tertiles (≤135.6), respectively. Left ventricular dysfunction was defined as ejection fraction ≤40%, and related variables were evaluated by backward conditional binary logistic regression analysis.
Results
The patients in the high PLR group were older (p<0.001) and had a higher rate of previous myocardial infarction and NSTEMI (p=0.046, p=0.013, respectively). There were significantly more coronary arteries narrowed (p=0.001) and lower left ventricular ejection fraction (p<0.001) in the high PLR group. Baseline platelet levels were significantly higher (p<0.001) and triglyceride and lymphocyte levels were significantly lower (p=0.009 and p<0.001, respectively) in the high PLR group. PLR >135.6 was found to be an independent predictor of systolic dysfunction in the multivariate analyses (ß: 0.306, 95% confidence interval: 0.151-0.619; p=0.001).
Conclusion
A high PLR is a strong and independent predictor for LVSD in patients with NST-ACS.
Keywords: acute coronary syndrome, myocardial infarction, platelet-lymphocyte ratio, ventricular ejection fraction
Introduction
Acute coronary syndrome (ACS) is a significant cause of morbidity and mortality in patients with coronary heart diseases in occidental countries. It is important to identify high-risk patients and to determine who will be treated immediately in ACS. About 40% of patients with an acute myocardial infarction (AMI) develop left ventricular systolic dysfunction (LVSD), whether or not there are signs of heart failure (HF) (1). Patients with HF and LVSD have a higher risk of adverse events than patients without LVSD or HF after AMI (2, 3). Additionally, previous studies demonstrated that elevated peripheral blood platelet count is associated with major adverse cardiovascular outcomes (4-7). On the contrary, a low peripheral blood lymphocyte count is related with major adverse cardiovascular outcomes (8-12). Therefore, a higher platelet-to-lymphocyte ratio (PLR) has emerged as a significant independent predictor of long-term survival in patients who present with non-ST elevation myocardial infarction (NSTEMI) (13). In this present study, we aimed to investigate the predictive value of PLR for left ventricular systolic dysfunction in patients with non-ST-elevated acute coronary syndrome (NST-ACS).
Methods
Study design
The present study was a retrospective study.
Study population
Records of patients with ACS, defined as unstable angina (UA) and NSTEMI, who were admitted to the coronary care unit of our department of cardiology in 18 Mart University, Faculty of Medicine between January 2011 and June 2012 were evaluated retrospectively. Patients with clinical evidence of cancer, active infection, hematological proliferative diseases, active or chronic inflammatory or autoimmune diseases, recent blood transfusion, severe hepatic diseases, and renal failure were excluded from this study. There were 35 patients excluded from the final analysis, 17 patients without WBC data, 14 patients with active infection, and 4 patients with a recent blood transfusion. Therefore, a total of 220 patients who were diagnosed with NST-ACS were included in the analysis in this study. The study protocol was approved by the local ethics committee of our hospital.
Study protocol
The study population was divided into tertiles based on admission PLR values. A high PLR group (n=73) was defined as having values in the third tertile (>135.6), and a low PLR group (n=147) was defined as having values in the lower 2 tertiles (≤135.6). Complete blood counts and biochemical values were evaluated retrospectively from blood samples obtained by ante- cubital vein puncture upon admission to the emergency department. Transthoracic echocardiography was performed on each patient immediately in the coronary care unit. Angiographic data of the patients were evaluated from catheter laboratory records.
Study variables
UA was diagnosed according to the following criteria: typical chest pain and/or electrocardiographic changes indicating myocardial ischemia with negative cardiac enzymes. An NSTEMI diagnosis was based on elevated cardiac enzymes with typical chest pain and/or electrocardiographic changes suggestive of myocardial ischemia. Typical chest pain was evaluated as follows: more than 20 minutes (min) in duration, new-onset angina, and an increase in its frequency and duration or severity. Demographic information, cardiovascular history, and risk factors, including smoking, hypertension (HT), and diabetes mellitus (DM), of patients were obtained from the medical records. Patients who had been treated with antihypertensive drugs or those whose baseline blood pressure exceeded 140/90 mm Hg were diagnosed with HT. DM was defined as fasting blood sugar of more than 126 mg/dL or the use of anti-diabetic medications. LVSD defined as ejection fraction ≤40%, measured by transthoracic echocardiography on first admission to coronary care unit.
Analysis of blood samples
Hematologic indices were evaluated from the CBC analysis performed with the Beckman Coulter LH 780 (Beckman Coulter Ireland Inc. Mervue, Galway, Ireland) in the hematology laboratory of our institution for all patients. Other biochemical measurements were determined by standard laboratory methods.
Echocardiography
All measurements were performed using a commercially available machine (Vivid 7®, GE Vingmed Ultrasound A/S, Horten, Norway) with a 3.5-MHz transducer for all patients. Simpson’s method was used to assess left ventricular ejection fraction (LVEF), as recommended by the American Society of Echocardiography (14).
Coronary angiography
All patients underwent a coronary angiography by the femoral approach using the standard Judkins technique. Iopromide, as a contrast agent (Ultravist-370, Bayer Schering Pharma, Germany), and a 6-F diagnostic catheter were used in all subjects. Diameter stenosis ≥70% with quantitative angiography was accepted as significant.
Statistical analysis
All statistical studies were carried out with the SPSS program (version 15.0, SPSS, Chicago, IL, USA). Quantitative variables were expressed as the mean value±standard deviation or median (interquartile range), and qualitative variables were expressed as percentages (%). Kolmogorov-Smirnov or Shapiro- Wilk test was done to determine normal distribution. A comparison of parametric values between the 2 groups was performed using the student t- or Mann-Whitney U test. Categorical variables were compared by the chi-square test or Fisher exact test. Spearman correlation analysis was used for the relation between PLR and LVEF. A backward conditional binary logistic regression analysis that included variables with p<.1 was performed to identify independent predictors of LVSD. Age ≥65, male gender, DM, heart rate ≥70, 2< vessels, NSTEMI, neutro- phil-to-lymphocyte ratio (NLR), and PLR >135.6 were entered into the model. A p value <0.05 was considered statistically significant.
Results
A total of 220 patients (162 men and 58 women) were enrolled in this present study. No significant differences were found between the low and high PLR groups regarding sex, heart rate, HT, DM, BMI, culprit lesion, and previous percutaneous coronary intervention (PCI). Table 1 demonstrates the baseline characteristics of the PLR groups. The patients in the high PLR group were older (66.1±11.3 vs. 59.7±11.9, p<0.001), had a lower rate of current smokers (27.4% vs. 43.5%, p=0.020), and had a higher rate of previous myocardial infarction (MI) and NSTEMI (15.1%, 6.8%, p=0.046, 69.9% vs. 52.4%, p=0.013, respectively). There were significantly more coronary arteries narrowed (1 and 3 vessels coronary arteries narrowed, p=0.001) and a lower LVEF [45 (25-65), 55 (25-65), p<0.001] in the high PLR group (Table 1).
Table 1.
The baseline characteristics of patients with low and high PLR
| Variable | Low PLR (≤135.6) n=147 | High PLR (>135.6) n=73 | P* |
|---|---|---|---|
| Gender, M/F | 110/37 | 52/21 | 0.569 |
| Age, years | 59.7±11.9 | 66.1±11.3 | 0.001 |
| Heart rate, beats per minute | 78 (46-115) | 80 (50-128) | 0.128 |
| Hypertension, % (n) | 53.7 (79) | 53.4 (39) | 0.965 |
| Diabetes mellitus, % (n) | 27.9 (41) | 30.1 (22) | 0.729 |
| Current smoker, % (n) | 43.5 (64) | 27.4 (20) | 0.020 |
| BMI, kg/m2 | 27.2 (17.3-42.5) | 27.7 (15.9-37.1) | 0.243 |
| Previous MI, % (n) | 6.8 (10) | 15.1 (11) | 0.046 |
| Previous PCI, % (n) | 10.9 (16) | 17.8 (13) | 0.153 |
| NSTEMI, % (n) | 52.4 (77) | 69.9 (51) | 0.013 |
| Number of coronary arteries narrowed, % (n) | 0.001 | ||
| 1 | 40.1 (59)** | 23.3 (17) | |
| 2 | 36.7 (54) | 28.8 (21) | |
| 3 | 23.1 (34)** | 47.9 (35) | |
| Culprit lesion, % (n) | 0.357 | ||
| LAD | 40.1 (59) | 49.3 (36) | |
| Cx | 36.1 (53) | 27.4 (20) | |
| RCA | 23.6 (35) | 23.3 (17) | |
| LV EF (%) | 55 (25-65) | 45 (25-65) | 0.001 |
BMI - body mass index; Cx - circumflex; LAD - left anterior descending; LVEF - left ventricular ejection fraction; MI - myocardial infarction; NSTEMI - non-ST elevation myocardial infarction; PCI - percutaneous coronary intervention; RCA - right coronary artery;
, Mann-Whitney U or student’s t-test for continuous variables and chi-square test for categorical variables;
; P<0.05 between two groups. p values in bold are significant
Baseline triglyceride [97 (35-360) vs. 120 (32-1950), p=0.009] and lymphocyte [1.4 (0.5-2.2) vs. 2.6 (0.8-12.2), p<0.001] levels were significantly lower, and platelet (282.4±63.1 vs. 228.2±60.3, p<0.001) levels and neutrophil-to-lymphocyte ratio (NLR) [4.18 (1.01-13.03), 2.67 (0.89-7.4), p<0.001] were significantly higher in the high PLR group (Table 2).
Table 2.
Patients’ laboratory findings
| Variable | Low PLR (≥135.6) n=147 | High PLR (>135.6) n=73 | P* |
|---|---|---|---|
| T cholesterol, mg/dL | 194 (98-445) | 186 (109-370) | 0.456 |
| LDL, mg/dL | 120 (110-186) | 121 (46-312) | 0.585 |
| HDL, mg/dL | 40(30-98) | 40 (21-88) | 0.930 |
| Triglyceride, mg/dL | 120 (32-1950) | 97 (35-360) | 0.009 |
| Hb, gr/dL | 12.8±1.5 | 12.4±1.6 | 0.063 |
| MPV | 8.77±0.99 | 8.51 ±0.90 | 0.059 |
| MCV, fL | 88.8 (14.8-107.1) | 88.1 (66.1-109.2) | 0.276 |
| PLT, 103/mm3 | 228.2±60.3 | 282.4±63.1 | 0.001 |
| WBC, 103/mm3 | 9.4 (3.8-19.0) | 9.0 (4.0-15.4) | 0.342 |
| Neutrophil, 103/mm3 | 5.6 (2.6-15.5) | 6.2 (1.3-13.0) | 0.059 |
| Lymphocyte, 103/mm3 | 2.6 (0.8-12.2) | 1.4 (0.5-2.2) | 0.001 |
| NLR | 2.67 (0.89-7.4) | 4.18 (1.01-13.03) | 0.001 |
| PLR | 89.0 (20.7-135.5) | 186.2 (135.7-724.3) | 0.001 |
Hb - hemoglobin; HDL - high-density lipoprotein; MPV - mean platelet volume; MCV - mean corpuscular volume; NLR - neutrophil-lymphocyte ratio; LDL - low-density lipoprotein; PLT - platelet; PLR - platelet-lymphocyte ratio; WBC - white blood cell;
, Mann-Whitney U or student’s t-test. p values in bold are significant
Table 3 demonstrates the baseline demographic and laboratory parameters of patients with and without LVSD. Patients with LVSD were older (67.2±11.5 vs. 60.3±11.8, p<0.001), had a significantly higher heart rate [81 (50-126) vs. 78 (44-115), p=0.028], and had a higher rate of previous MI and NSTEMI (20%, 6.5%, p=0.004, 80% vs. 51.8%, p<0.001, respectively). Platelet and neutrophil levels and NLR were significantly higher [288.1±57.5 vs. 233.9±63.7, p<0.001, 6.6 (1.3-12.0) vs. 5.7 (2.6-15.5), p=0.043, 3.63 (1.68-10.75), 2.57 (0.89-13.03), p<0.001, respectively], lymphocyte levels were significantly lower [1.7 (0.5-3.9) vs. 2.2 (0.7-12.2), p=0.001], and PLR was significantly increased [164.2 (66.3-724.3) vs. 101.5 (20.7-334.4), p<0.001] in patients with LVSD. PLR was negatively correlated with LVEF in the correlation analysis (r=- 0.400, p<0.001). The relationship between LVEF and PLR in NST- ACS patients is demonstrated in Figure 1.
Table 3.
Comparison of patients with and without left ventricular systolic dysfunction
| Variable | EF≤40% (n=50) | EF >40% (n=170) | P* |
|---|---|---|---|
| Male (M/F) | 35/15 | 127/43 | 0.507 |
| Age, years | 67.2±11.5 | 60.3±11.8 | 0.001 |
| Heart rate, bmp | 81 (50-126) | 78 (44-115) | 0.028 |
| Hypertension, % (n) | 46.0 (23) | 55.9 (95) | 0.218 |
| Diabetes mellitus, % (n) | 38.0 (19) | 25.9 (44) | 0.096 |
| Current smoker, % (n) | 30.0 (15) | 40.6 (69) | 0.176 |
| T cholesterol, mg/dL | 190 (109-445) | 193 (98-441) | 0.518 |
| LDL, mg/dL | 120 (46-224) | 120 (110-312) | 0.789 |
| Triglyceride, mg/dL | 103 (41-1450) | 116 (32-673) | 0.114 |
| HDL, mg/dL | 38 (28-98) | 40 (30-83) | 0.850 |
| PLT, 103/mm3 | 288.1±57.5 | 233.9±63.7 | 0.001 |
| MCV, fL | 88.3 (64.0-109.2) | 89.6 (64.8-104.4) | 0.581 |
| MPV | 8.8±1.0 | 8.6±0.9 | 0.178 |
| Hb | 12.4± 1.6 | 12.7±1.5 | 0.295 |
| WBC | 9.9 (5.0-15.9) | 9.1 (3.8-19.0) | 0.098 |
| Neutrophil (103/mm3) | 6.6 (1.3-12.0) | 5.7 (2.6-15.5) | 0.043 |
| Lymphocyte (103/mm3) | 1.7 (0.5-3.9) | 2.2 (0.7-12.2) | 0.001 |
| NLR | 3.63 (1.68-10.75) | 2.57 (0.89-13.03) | 0.001 |
| LVEF (%) | 35 (25-40) | 55 (44-65) | 0.001 |
| NSTEMI, % (n) | 80.0 (40) | 51.8 (88) | 0.001 |
| Previous MI, % (n) | 6.5 (11) | 20.0 (10) | 0.004 |
| Number of coronary arteries narrowed | 0.065 | ||
| 1 | 24.0 (12) | 37.6 (64) | |
| 2 | 32.0 (16) | 34.7 (59) | |
| 3 | 44.0 (22) | 27.6 (47) | |
| Culprit lesion | 0.287 | ||
| LAD | 50.0 (25) | 41.2 (70) | |
| Cx | 24.0 (12) | 35.9 (61) | |
| RCA | 26.0 (13) | 22.9 (39) | |
| PLR | 164.2 (66.3-724.3) | 101.5 (20.7-334.4) | 0.001 |
bmp - beats per minute; Cx - circumflex; Hb - hemoglobin; HDL - high-density lipoprotein; MPV - mean platelet volume; MCV - mean corpuscular volume; LAD - left anterior descending; LDL - low-density lipoprotein; LVEF - left ventricular ejection fraction; MI - myocardial infarction; NLR - neutrophil-lymphocyte ratio; NSTEMI - non- ST elevation myocardial infarction; PLT - platelet; PLR - platelet-lymphocyte ratio; RCA - right coronary artery; WBC - white blood cell;
Mann-Whitney U or student’s t-test for continuous variables and chi-square test for categorical variables. p values in bold are significant
Figure 1.
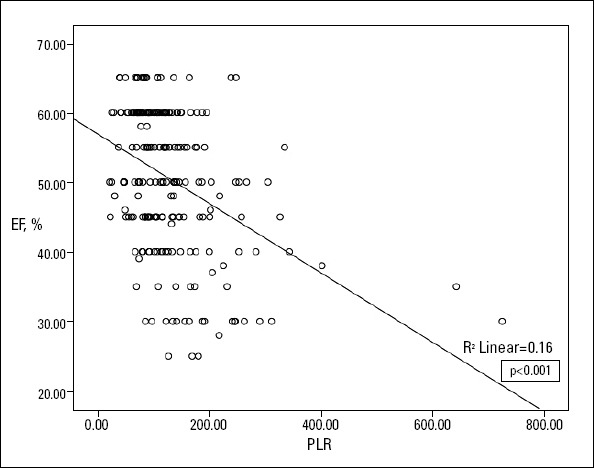
Relationship between left ventricular ejection fraction (EF) and platelet-lymphocyte ratio (PLR) in non-ST elevated acute coronary syndrome (NST-ACS) patients
Independent predictors of systolic dysfunction were determined by backward stepwise multivariate logistic regression. Age ≥65, NSTEMI, previous MI, NLR, and PLR >135.6 were found to be associated with systolic dysfunction (Table 4). PLR >135.6, age ≥65, and previous MI and NSTEMI were found to be independent predictors of systolic dysfunction in the multivariate analyses (β: 2.88, 95% CI: 1.39-5.95, p=0.004; β: 2.45, 95% CI: 1.17-5.11, p=0.017; β: 0.20, 95% CI: 0.07-0.60, p=0.004; β: 4.18, 95% CI: 1.77-9.87, p=0.001; respectively, Table 5).
Table 4.
Univariate analysis for risk factors of left ventricular systolic dysfunction
| Variable | β (95% CI) | P |
|---|---|---|
| DM | 0.57 (0.29-1.10) | 0.098 |
| Male | 1.26 (0.63-2.54) | 0.507 |
| Heart rate ≥70 | 1.76 (0.82-3.79) | 0.147 |
| 2≤ vessels | 1.91 (0.93-3.92) | 0.077 |
| Age ≥65 years | 2.84 (1.46-5.50) | 0.002 |
| NLR | 1.31 (1.13-1.51) | 0.001 |
| High PLR | 4.43 (2.28-8.590.438) | 0.001 |
| NSTEMI | 3.72 (1.75-7.93) | 0.001 |
| Previous MI | 0.27 (0.11-0.69) | 0.006 |
CI - confidence interval; DM - diabetes mellitus; HR - hazard ratio; MI - myocardial infarction; NLR - neutrophil-lymphocyte ratio; NSTEMI - non-ST elevation myocardial infarction; PLR - platelet-lymphocyte ratio. p values in bold are significant
Table 5.
Multivariate analysis for risk factors of left ventricular systolic dysfunction
| Variable | β (95% CI) | P |
|---|---|---|
| Age ≥65 years | 2.45 (1.17-5.11) | 0.017 |
| High PLR | 2.88 (1.39-5.95) | 0.004 |
| NSTEMI | 4.18 (1.77-9.87) | 0.001 |
| Previous MI | 0.20 (0.07-0.60) | 0.004 |
CI - confidence interval; HR - hazard ratio; MI - myocardial infarction; NSTEMI - non- ST elevation myocardial infarction; PLR - platelet-lymphocyte ratio. p values in bold are significant
Discussion
The present study shows that PLR ≥135.6 was associated with LVEF ≤40% on admission in patients with NST-ACS. Why should we determine patients with LVSD in ACS? For a long time, it has been known that not only is ACS related to mortality but also morbidity, especially congestive HF, and when HF occurs together, coronary artery disease have higher rate of mortality.
Platelets play an important role in the progression of atherosclerosis. Some studies showed that platelets interact with endothelial cells and leukocytes and precipitate the release of inflammatory substances that lead to adhesion and transmigration of monocytes (15, 16). These monocytes increase the inflammatory processes in the vessel wall, promoting atherosclerotic lesions (17). Furthermore, platelets both promote atherogenesis and start its complications (4). In addition, inflammation was also shown to inhibit collateral formation, mainly by affecting endothelial function (18). Moreover, Alexandrakis et al. (19) demonstrated that low-grade inflammation may increase circulating platelet count, which reflects the underlying inflammation and several inflammatory mediators that cause thrombocytosis. Thrombocytosis is commonly associated with coronary artery disease and has been widely reported as a poor prognostic marker (4-6). Stissing et al. (20) reported that patients with pathologically increased platelet counts have an elevated risk of thrombotic complications.
Also, Ommen et al. (12) have documented a decrease in the total and relative numbers of circulating lymphocytes during AMI and advanced congestive HF. The primary mechanism behind this is speculative. It has been proposed that in response to physiologic stress during myocardial ischemia/infarction, there is a release of cortisol. High cortisol leads to lymphopenia (21). Lazaro et al. (22) showed that inflammatory markers are related to functional class and prognosis in stable HF patients, and they concluded that inflammation plays an essential role in HF. Lymphopenia, in view of lymphocyte apoptosis, shows a high inflammatory process (23), because lymphopenia occurs due to lymphocyte apoptosis and pro-inflammatory cytokines released due to apoptotic cells in acute situations (24). Blum et al. (25) reported that lymphopenia and low CD4 count vigorously correlated with low ejection fraction and high myocardial mass destruction.
Therefore, high NLR has been demonstrated as a sign of a high inflammatory process and an independent predictor for LVSD in CAD and HF in recent studies (12, 26). Similarly, NLR was higher in patients with LVSD in our study. But, in the multivariate regression analysis, we found that PLR was significant and NLR was not. These findings suggest that PLR was a stronger predictor in LVSD in our study.
Eventually, both thrombocytosis and lymphopenia correlate with the degree of systemic inflammation, and PLR indicates a new marker incorporating both hematologic indices. These possible mechanisms can explain why a higher PLR is independently related with LVSD, while multi-vessel disease is not. The increased inflammatory response appears to be mediated by greater myocardial damage in ACS patients. Therefore, in this study, we have proposed that the inflammatory process, although not directly, may be strongly associated with cardiovascular outcomes.
Similar to previous studies conducted in this area, our patients were significantly older in the high PLR group. To our knowledge, the relationship between age and PLR has not been investigated in the literature so far. However, in our experience, no study has been performed on the relationship between PLR and systolic dysfunction in patients NST-ACS. In the present study, we demonstrated a relationship between systolic dysfunction and mean PLR values.
Study limitations
This present study has some limitations. Firstly, this was retrospective and based on a relatively small group of patients and was a single-center study. Secondly, the LVEF of patients before their admission to the hospital was not known. Thirdly, PLR values may be relevant in some conditions, such as platelet aggregation and inflammatory markers. In this study, these parameters were not measured. Fourthly, GRACE and TIMI risk scores were not calculated. Finally, coronary blush grade, no reflow, and extent of coronary thrombus parameters were not analyzed in the coronary angiography.
Conclusion
A high PLR is a strong and independent predictor for LVSD in patients with NST-ACS. Platelet and lymphocyte count analyses are routine applications and simple and inexpensive methods for evaluating patients with ACS. PLR and clinical findings might be helpful to determine high-risk patients and treatment strategies.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept - A.B., E.G., M.Y.; Design - A.B., E.G., M.Y.; Supervision - A.B., E.G., M.Y., T.P.; Resource - A.B., M.Y., T.P., A.T.; Materials - A.B., T.P.; Data collection &/or processing - A.B., A.T., A.Barutçu.; Analysis &/or interpretation - A.B., A.Barutçu., B.A.; Literature search - A.B., B.A., A.T.; Writing - A.B., E.G., A.B.; Critical review - A.B., M.Y., B.A.; Other - A.Barutçu.
References
- 1.Køber L, Torp-Pedersen C, Jørgensen S, Eliasen P, Camm AJ. Changes in absolute and relative importance in the prognostic value of left ventricular systolic function and congestive heart failure after acute myocardial infarction. TRACE study group. Am J Cardiol. 1998;81:1292–7. doi: 10.1016/s0002-9149(98)00158-1. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 2.Hasdai D, Topol EJ, Kilaru R, Battler A, Harrington RA, Vahanian A, et al. Frequency, patient characteristics, and outcomes of mild-to- moderate heart failure complicating ST-segment elevation acute myocardial infarction:lessons from 4 international fibrinolytic therapy trials. Am Heart J. 2003;145:73–9. doi: 10.1067/mhj.2003.53. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 3.Steg PG, Dabbous OH, Feldman LJ, Cohen-Solel A, Aumont MC, Lopez-Sendon J, et al. Determinants and prognostic impact of heart failure complicating acute coronary syndromes:observation from GRACE.“. Circulation. 2004;109:494–9. doi: 10.1161/01.CIR.0000109691.16944.DA. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 4.Thaulow E, Erikssen J, Sandvik L, Stormorken H, Cohn PF. Blood platelet count and function are related to total and cardiovascular death in apparently healthy men. Circulation. 1991;84:613–7. doi: 10.1161/01.cir.84.2.613. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 5.Iijima R, Ndrepepa G, Mehilli J, Bruskina O, Schulz S, Schomig A, et al. Relationship between platelet count and 30 day clinical outcomes after percutaneous coronary interventions. Pooled analysis of four ISAR trials. Thromb Haemost. 2007;98:852–7. [PubMed] [Google Scholar]
- 6.Nikolsky E, Grines CL, Cox DA, Garcia E, Tcheng JE, Sadeghi M, et al. Impact of baseline platelet count in patients undergoing primary percutaneous coronary intervention in acute myocardial infarction (from the CADILLAC trial) Am J Cardiol. 2007;99:1055–61. doi: 10.1016/j.amjcard.2006.11.066. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 7.Jakl M, Sevcik R, Ceral J, Fatorova I, Horacek JM, Vojacek J. Mean platelet volume and platelet count:overlooked markers of high on- treatment platelet reactivity and worse outcome in patients with acute coronary syndrome. Anadolu Kardiyol Derg. 2014;14:85–6. doi: 10.5152/akd.2013.4803. [DOI] [PubMed] [Google Scholar]
- 8.Ommen SR, Hammill SC, Gibbons RJ. The relative lymphocyte count predicts death in patients receiving implantable cardioverter defibrillators. Pacing Clin Electrophysiol. 2002;25:1424–8. doi: 10.1046/j.1460-9592.2002.01424.x. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 9.Acanfora D, Gheorghiade M, Trojano L, Furgi G, Pasini E, Picone C, et al. Relative lymphocyte count:a prognostic indicator of mortality in elderly patients with congestive heart failure. Am Heart J. 2001;142:167–73. doi: 10.1067/mhj.2001.115792. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 10.Zouridakis EG, Garcia-Moll X, Kaski JC. Usefulness of the blood lymphocyte count in predicting recurrent instability and death in patients with unstable angina pectoris. Am J Cardiol. 2000;86:449–51. doi: 10.1016/s0002-9149(00)00963-2. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 11.Ommen SR, Gibbons RJ, Hodge DO, Thomson SP. Usefulness of the lymphocyte concentration as a prognostic marker in coronary artery disease. Am J Cardiol. 1997;79:812–4. doi: 10.1016/s0002-9149(96)00878-8. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 12.Ommen SR, Hodge DO, Rodeheffer RJ, McGregor CG, Thomson SP, Gibbons RJ. Predictive power of the relative lymphocyte concentration in patients with advanced heart failure. Circulation. 1998;97:19–22. doi: 10.1161/01.cir.97.1.19. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 13.Azab B, Shah N, Akerman M, McGinn JT., Jr Value of platelet/ lymphocyte ratio as a predictor of all-cause mortality after non-ST elevation myocardial infarction. J Thromb Thrombolysis. 2012;34:326–34. doi: 10.1007/s11239-012-0718-6. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;2:79–108. doi: 10.1016/j.euje.2005.12.014. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 15.Gawaz M, Langer H, May AE. Platelets in inflammation and athero- genesis. J Clin Invest. 2005;115:3378–84. doi: 10.1172/JCI27196. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindemann S, Kramer B, Seizer P, Gawaz M. Platelets, inflammation and atherosclerosis. J Thromb Haemost. 2007;5:203–11. doi: 10.1111/j.1538-7836.2007.02517.x. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 17.Huo Y, Schober A, Forlow SB, Smith DF, Hyman MC, Jung S, et al. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003;9:61–7. doi: 10.1038/nm810. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 18.Schneeweis C, Gräfe M, Bungenstock A, Spencer-Hänsch C, Fleck E, Goetze S. Chronic CRP-exposure inhibits VEGF-induced endothelial cell migration. J Atheroscler Thromb. 2010;17:203–12. doi: 10.5551/jat.3004. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 19.Alexandrakis MG, Passam FH, Moschandrea IA, Christophoridou AV, Pappa CA, Coulocheri SA, et al. Levels of serum cytokines and acute phase proteins in patients with essential and cancer-related thrombocytosis. Am J Clin Oncol. 2003;26:135–40. doi: 10.1097/00000421-200304000-00007. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 20.Stissing T, Dridi NP, Ostrowski SR, Bochsen L, Johansson PI. The influence of low platelet count on whole blood aggregometry assessed by Multiplate. Clin Appl Thromb Hemost. 2011;17:211–7. doi: 10.1177/1076029610397183. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 21.Thomson SP, McMahon LJ, Nugent CA. Endogenous cortisol:a regulator of the number of lymphocytes in peripheral blood. Clin Immunol Immunopathol. 1980;17:506–14. doi: 10.1016/0090-1229(80)90146-4. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 22.Sánchez-Lázaro IJ, Almenar L, Reganon E, Vila V, Martınez-Dolz L, Martinez-Sales V, et al. Inflammatory markers in stable heart failure and their relationship with functional class. Int J Cardiol. 2008;129:388–93. doi: 10.1016/j.ijcard.2007.07.138. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 23.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–50. doi: 10.1056/NEJMra021333. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 24.Gupta S, Agrawal A, Agrawal S, Su H, Gollapudi S. A paradox of immunodeficiency and inflammation in human aging:lessons learned from apoptosis. Immun Ageing. 2006;3:5. doi: 10.1186/1742-4933-3-5. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blum A, Sclarovsky S, Rehavia E, Shohat B. Levels of T-lymphocyte subpopulations, interleukin-1 beta, and soluble interleukin-2 receptor in acute myocardial infarction. Am Heart J. 1994;127:1226–30. doi: 10.1016/0002-8703(94)90040-x. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 26.Doğdu O, Akpek M, Yarlıoğlueş M, Kalay N, Ardıç I, Elçik D, et al. Relationship between hematologic parameters and left ventricular systolic dysfunction in stable patients with multi-vessel coronary artery disease. Turk Kardiyol Dern Ars. 2012;40:706–13. doi: 10.5543/tkda.2012.82429. [CrossRef] [DOI] [PubMed] [Google Scholar]


