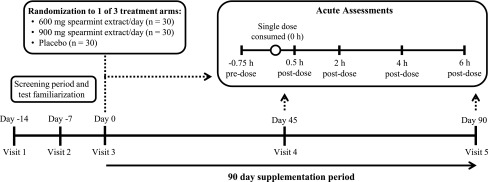
FIG. 1.
Study design overview. This double-blind, placebo-controlled, parallel study included one telephone screen, two screening visits (days −14 and −7), a baseline visit (day 0), and two treatment visits (days 45 and 90). Subjects were randomly assigned one of three treatments: 600 mg spearmint extract, 900 mg spearmint extract, or placebo, which was consumed each day with breakfast over a 90-day treatment period. During baseline and treatment visits, subjects completed the CDR System™ computerized cognitive function test battery (days 0, 45, and 90, at −0.75, 0.5, 2, 4, and 6 h), computerized Profile of Mood States (days 0 and 90 only; at −0.75 h), and computerized Leeds Sleep Evaluation Questionnaire (days 0 and 90 only; at −0.75 h). After their predose assessments, subjects consumed a standardized breakfast and one dose of their assigned study product (0 h) followed by postdose computerized CDR test battery. CDR, Cognitive Drug Research. Figure modified from Lasrado et al.27 and reproduced with permission.