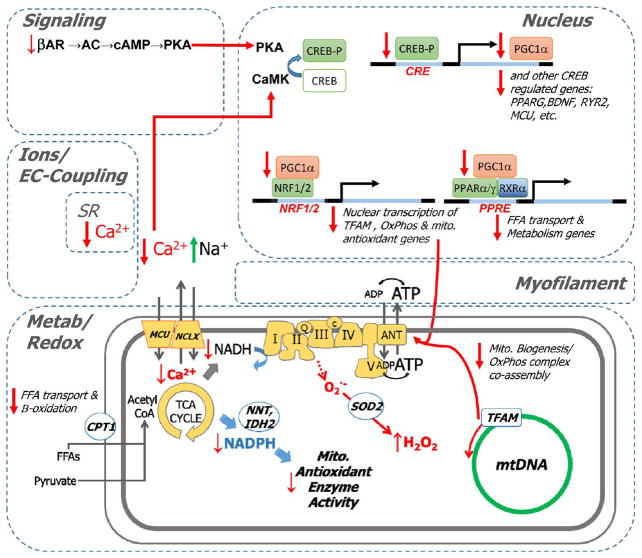
Figure 11.
Model for excitation–transcription coupling responsible for maladaptive metabolic and antioxidant remodeling in HF. Deficits in β-adrenergic signaling and Ca2+ handling are hallmarks of HF that are recapitulated in the guinea pig model. These deficits are expected to diminish the activity of protein kinase A (PKA) and Ca2+ calmodulin-dependent kinases. Pathway analysis suggests an analogy to neuronal signaling, in which PKA and CAMK activity have been shown to govern the phosphorylation state and activation status of the cAMP-responsive element (CRE) binding protein (CREB), a transcriptional coactivator. Since CREB is a documented transcriptional coactivator of PGC1α (itself a coactivator) and other genes (e.g., RYR2, BDNF), impaired CREB activation by CaMK/PKA would impinge on PGC1α-dependent gene programs, among them PPAR/RXR-mediated activation of genes for fatty acid metabolism (e.g., CPT1B, β-oxidation enzymes). PGC1α also participates in NRF 1- and 2-mediated activation of genes responsible for mitochondrial homeostasis (e.g., TFAM) and antioxidant function (e.g., SOD2 and PRDX3). Finally, antioxidant defenses suffer a double blow. Not only are antioxidant proteins downregulated, but also, acute Ca2+/Na2+ dysregulation abrogates mitochondrial TCA-cycle-dependent NADPH production, which is required to sustain the activity of thiol-bearing antioxidant enzymes.