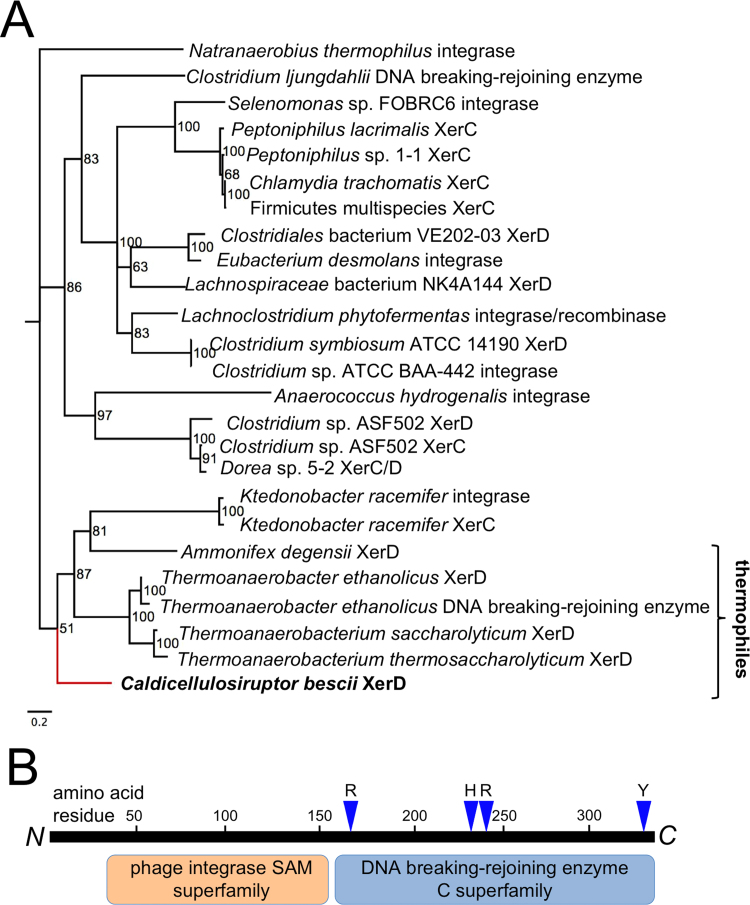
Fig. 2.
Cbes2777 has thermophilic homologs and a conserved Xer-like catalytic domain. (A) Maximum likelihood tree of Xer-like homologs of Cbes2777. Cbes2777 resides in a cluster with predominantly thermophilic organisms (Topt≥60 °C), indicated by the bracket. Posterior probabilities determined by MRBAYES are shown at the nodes of the tree. The XerD homolog from Caldicellulosiruptor bescii is indicated by a red branch. The scale bar indicates the distance for 0.2 amino acid substitutions per site. (B) Protein domains of the Cbes2777 XerD recombinase. R164, H243, R246, and Y328 appear to constitute the RHRY conserved catalytic residues (Subramanya et al., 1997), indicated above the C-terminal catalytic domain. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)