Figure 1.
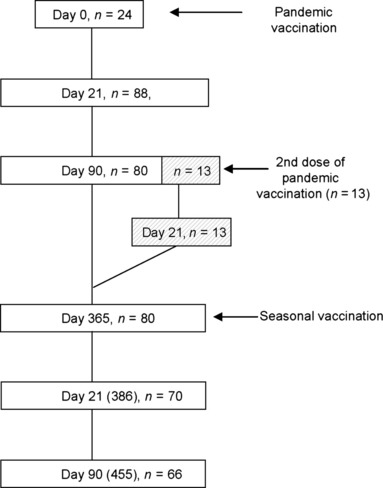
A schematic presentation of study design. The study included 96 clinically healthy healthcare professionals aged 20–64 years. The subject received monovalent Pandemrix vaccination at day 0 and follow‐up samples were collected at days 21, 90, and 365. A subgroup of vaccinees (n = 13), who were low responders to the initial Pandemrix vaccination received a second dose at day 90 and an additional follow‐up sample was collected 21 days later. At day 365, all subjects received a second trivalent non‐adjuvanted seasonal influenza vaccine and follow‐up samples were collected at days 21 and 90 after the booster vaccination. The numbers at each time point indicate the number of subjects of whom serum samples were collected.
