Abstract
GABAergic projections terminate on numerous hippocampal interneurons containing calcium binding proteins (CBPs), including calbindin D-28k (CB), calretinin (CR) and parvalbumin (PV). Memory deficits and expression levels of CB, CR, and PV were examined in the hippocampal subregions following systemic scopolamine (Scop; 1 mg/kg) treatment for 4 weeks in mice. Scop treatment induced significant memory deficits from 1 week after Scop treatment. CB, CR and PV immunoreactivities distributions were in hippocampal subregions [CA1 and CA3 regions, and the dentate gyrus (DG)]. CB immunoreactivity (CB+) was gradually decreased in all subregions until 2 weeks after Scop treatment, and CB+ was decreased to the lowest level in all subregions at 3 and 4 weeks. CR+ in the CA1 region was gradually decreased until 2 weeks and hardly observed at 3 and 4 weeks; in the CA3 region, CR+ was not altered in all subregions at any time. In the DG, CR+ was gradually decreased until 2 weeks and lowest at 3 and 4 weeks. PV+ in the CA1 region was not altered at 1 week, and gradually decreased from 2 weeks. In the CA3 region, PV+ did not change in any subregions at any time. In the DG, PV+ was not altered at 1 week, decreased at 2 weeks, and lowest at 3 and 4 weeks. In brief, Scop significantly decreased CBPs expressions in the hippocampus ≥3 weeks after the treatment although memory deficits had developed at 1 week. Therefore, it is suggested that Scop (1 mg/kg) must be systemically treated for ≥3 weeks to investigate changes in expression levels of CBPs in the hippocampus.
Keywords: scopolamine, memory impairments, calcium binding proteins, CA1-3 region, dentate gyrus
Introduction
Muscarinic acetylcholine receptors (mAChRs) are primary receptors in cholinergic transmission, and mAChRs immunoreactivities in the hippocampus are present in pyramidal cells, granule cells, and GABAergic interneurons (1). It is well established that systemic injection of scopolamine (Scop), a muscarinic cholinergic receptor antagonist, produces learning and memory deficits through dysregulation of cholinergic mechanisms in the hippocampus (2).
Septo-hippocampal cholinergic pathway, which originates in the medial septum/diagonal band, is one of crucial circuits for cognitive function (3). Also, it is well known that septo-hippocampal GABAergic pathway plays important roles in learning and memory processes (4). Important inputs to the hippocampus are provided by GABAergic fibers as well as cholinergic fibers from the medial septum, and most of GABAergic projections terminate on many hippocampal interneurons including neurons containing calcium binding proteins (CBPs), such as calbindin D-28k (CB), calretinin (CR), and parvalbumin (PV) (5–7). GABAergic nonpyramidal cells transmit hippocampal feedback to GABAergic neurons in the medial septum, which forms septo-hippocampo-septal circuit that regulates the activity of GABAergic septo-hippocampal projections (8).
The maintenance of intracellular calcium metabolism in the hippocampus is an important factor in synaptic plasticity and memory performance (9,10). Alterations in numbers or expressions of CBPs in hippocampal neurons are observed in post-mortem brain tissues in schizophrenia (11) and kainate-induced seizures (12), and these changes contribute to cognitive impairments in neurological disorders (13,14). However, in Scop-induced amnesia models, alterations in CBPs expressions have not been fully studied.
In the present study, therefore, we examined temporal and spatial alterations of CBPs expressions in hippocampal subregions after chronic systemic treatment with Scop in mice, and investigated the relationship between hippocampal CBPs expressions and memory deficits after Scop treatment.
Materials and methods
Experimental animals
Male ICR mice (8-week-old) were purchased from Orient Bio Inc. (Seongnam, South Korea) and used after 7 days of acclimation. The procedures for animal handling and care adhered to guidelines that are in compliance with the current international laws and policies (Guide for the Care and Use of Laboratory Animals, The National Academies Press, 8th edition, 2011), and the experimental protocol of the present study was reviewed and approved based on ethical procedures and scientific care by the Kangwon National University-Institutional Animal Care and Use Committee (approval no. KW-160802-3).
Experimental groups and drug treatment
Experimental mice were divide into five groups (n=14 at each point in time per group): i) Control group; ii) 1 week (wk)-Scop group, which received Scop treatment for 1 week; iii) 2 wk-Scop group; iv) 3 wk-Scop group; and v) 4 wk-Scop group. Normal saline or 1 mg/kg Scop was dissolved in normal saline and intraperitoneally injected once a day for 1 to 4 weeks (15–17). The mice in each group were sacrificed 24 h after the final administration.
Behavioral performance
Morris water maze test (MWMT)
The MWMT for spatial memory was performed according to our published procedure (18). The animals (n=7) in each group were tested 1 day before sacrifice following training for 3 days to find the platform. Shortly, the time to find the platform within 120 sec (latency time), and the time in the target quadrant were recorded in each animal. The whole process was monitored by a digital camera and a computer system.
Passive avoidance test (PAT)
PAT for short-term memory was performed according to our published method (19). The animals (n=7) in each group were tested 1 day before sacrifice following training for 1 day. Briefly, we used the Gemini Avoidance system (GEM 392; San Diego Instruments, San Diego, CA, USA) that consists of light and dark compartments with a grid floor. For training, the animals in each group were allowed to explore environments in both light and dark compartments for 1 min and given an inescapable foot-shock (0.3 mA for 3 sec) after entering the dark compartment. The animals were tested 15 min after the training without applying the foot-shock. The interval between the beginning of the test and the entry into the dark compartment was defined as the latency time.
Immunohistochemistry
Immunohistochemistry for CB, CR, and PV was carried out according to our published method (20). Briefly, mice (n=7 in each group) were anesthetized with 30 mg/kg Zoletil 50 (Virbac, Carros, France) and perfused transcardially with 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4). Their brains were serially sectioned into 30-µm coronal sections in a cryostat (Leica Microsystems GmbH, Wetzlar, Germany). The sections were incubated with diluted goat anti-CB (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-CR antibody (1:1,000; Chemicon International, Inc., Temecula, CA, USA) or goat anti-PV (1:200; Santa Cruz Biotechnology) overnight and subsequently exposed to biotinylated rabbit anti-goat or goat anti-rabbit IgG, and streptavidin peroxidase complex (1:200; both from Vector Laboratories, Inc., Burlingame, CA, USA). In order to establish the specificity of the immunostaining, a negative control resulted in the absence of immunoreactivity in the stained sections.
Data analysis
As previously described (19), digital images of CB-, CR-, and PV-immunoreactive structures were captured with an AxioM1 light microscope equipped with a digital camera (Axiocam; both from Carl Zeiss AG, Oberkochen, Germany) connected to a PC monitor. The density of each immunoreactive structures was evaluated on the basis of optical density (OD), which was obtained after the transformation of the mean gray level using the formula: OD=log (256/mean gray level). After the background was subtracted, a ratio of the OD of image file was calibrated as % [relative optical density (ROD)] using Adobe Photoshop version 8.0 and NIH Image J software (National Institutes of Health, Bethesda, MD, USA). The mean value of the OD of the control group was designated as 100%, and the ROD of each group was calibrated and expressed as % of the control group.
Statistical analysis
The data shown here represent the means ± SEM. Differences of the means among the groups were statistically analyzed by analysis of variance with a post hoc Bonferroni's multiple comparison test in order to elucidate effects of long-term Scop treatment among groups. Statistical significance was considered at P<0.05.
Results
MWMT
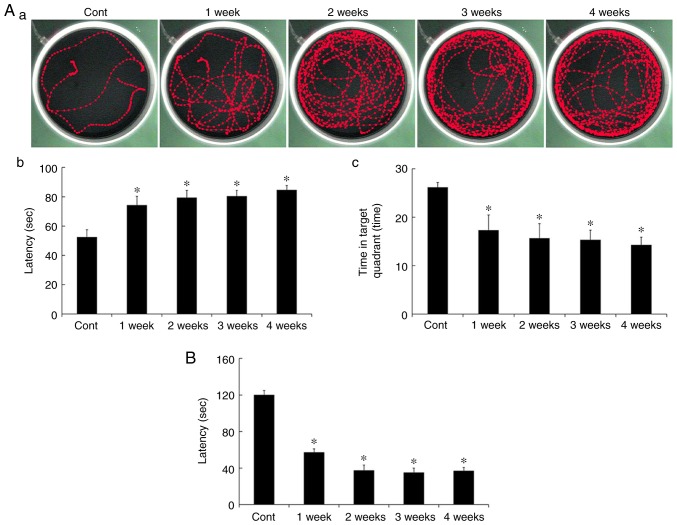
Spatial memory was evaluated using the MWMT. Escape latency in all the Scop treated groups was significantly increased compared to that in the control group (Fig. 1Aa and Ab). In addition, duration of the time in the target quadrant was significantly decreased in all the Scop treated groups (Fig. 1Aa and Ac). However, there were no significant differences among the Scop treated groups (Fig. 1Ab and Ac).
Figure 1.
Memory test using the MWMT (Aa-Ac) and PAT (B) in the control and Scop groups. In the MMWT, escape latency in all the Scop groups is significantly increased compared with that in the control group (Aa and Ab), and duration of the time in the target quadrant in all the Scop groups is significantly decreased compared with that in the control group (Ac). In the PAT, latency in all the Scop groups is significantly decreased compared with that in the control group (B). (n=7 per group; *P<0.05 vs. the control group). The bars indicate the SEM. MWMT, Morris water maze test; PAT, passive avoidance test; Scop, scopolamine.
PAT
Short-term memory was evaluated using PAT. Similar to results of the MWMT, latency in all the Scop treated groups was significantly decreased compared to that in the control group (Fig. 1B), however, the latency was not significantly different among the Scop treated groups (Fig. 1B).
CB immunoreactivity
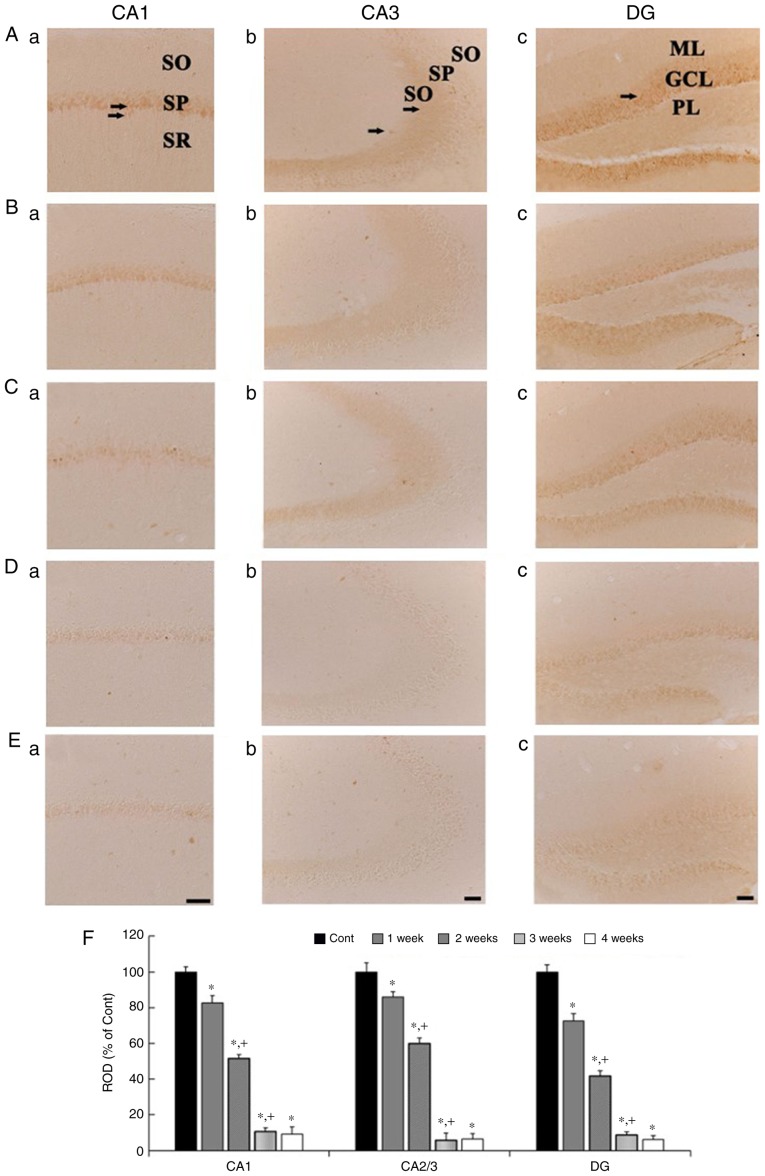
CB immunohistochemistry results across weeks 1 to 4 are presented in Fig. 2. In the control group, CB immunoreactivity was identified in neurons of the stratum pyramidale (SP) in the CA1 region, in mossy fibers of the stratum lucidum in the CA3 region, and in cells of the granule cell layer (GCL) in the dentate gyrus (DG) (Fig. 2Aa-Ac). In the 1 wk-Scop group, the distribution pattern of CB immunoreactivity in all the subregions was similar to that in control group, however, the CB immunoreactivity in all the subregions was significantly decreased compared to that in the control group (Fig. 2Ba-Bc and F). In the 2 wk-Scop group, CB immunoreactivity was more reduced compared with that in the 1 wk-Scop group (Fig. 2Ca-Cc and F). In the 3 wk- and 4 wk-Scop groups, CB immunoreactivity was hardly shown in the CA1 and CA3 regions (Fig. 2Da, Db, Ea, Eb and F), and very weak in the DG (Fig. 2Dc, Ec and F).
Figure 2.
CB immunohistochemistry in the hippocampal subregions of the control (Aa-Ac), 1 wk-(Ba-Bc), 2 wk-(Ca-Cc), 3 wk-(Da-Dc), and 4 wk-(Ea-Ec) Scop groups. CB immunoreactivity in the control group is shown in cells the stratum pyramidale (SP, arrows) in the CA1 region, in the mossy fibers (arrows) in the CA3 region, and cells in the granule cell layer (GCL, arrow) in the DG. CB immunoreactivity in the Scop groups is gradually and significantly decreased with time in all the subregions. Scar bar, 50 µm. (F) ROD of CB immunoreactive structures in the control and Scop groups (n=7 per group; *P<0.05 vs. the control group; †P<0.05 vs. the pre-time point Scop group). The bars indicate the SEM. CB, calbindin D-28k; wk, week; Scop, scopolamine; SP, stratum pyramidale; GCL, granule cell layer; DG, dentate gyrus; ML, molecular layer; PL, polymorphic layer; SO, stratum oriens; SR, stratum radiatum; ROD, relative optical density.
CR immunoreactivity
CR immunohistochemistry results across weeks 1 to 4 are presented in Fig. 3. In the control group, CR immunoreactivity in the CA1 and CA3 regions was shown in cells scattered in the strata oriens and radiatum (Fig. 3Aa and Ab), and, in the DG, CR immunoreactivity is found in the GCL and cells scatted in the polymorphic layer (PL) (Fig. 3Ac). In the Scop groups, CR immunoreactivity was gradually decreased with time in the CA1 region (Fig. 3Ba, Ca, Da, Ea and F), not significantly altered in the CA3 region (Fig. 3Bb, Cb, Db, Eb and F). In the DG, CR immunoreactivity was gradually decreased, in particular, CR immunoreactivity in the 3- and 4-wk Scop groups was shown in a few cells in the PL (Fig. 3Bc, Cc, Dc, Ec and F).
Figure 3.
CR immunohistochemistry in the hippocampal subregions of the control (Aa-Ac), 1 wk-(Ba-Bc), 2 wk-(Ca-Cc), 3 wk-(Da-Dc), and 4 wk-(Ea-Ec) Scop groups. CR immunoreactivity in the control group is shown cells (arrows) scattered in the CA1 and CA3 region, and cells in the granule cell layer (GCL, arrow) and cells in the polymorphic layer (PL, arrow) in the DG. CR immunoreactivity is significantly reduced in the CA1 region and the DG with time after Scop treatment, but not in the CA3 region. Scar bar, 50 µm. (F) ROD of CR immunoreactive structures in the control and Scop groups (n=7 per group; *P<0.05 vs. the control group; †P<0.05 vs. the pre-time point Scop group). The bars indicate the SEM. CR, calretinin; wk, week; Scop, scopolamine; SO, stratum oriens; SP, stratum pyramidale; SR, stratum radiatum; GCL, granule cell layer; PL, polymorphic layer; ML, molecular layer; DG, dentate gyrus; ROD, relative optical density.
PV immunoreactivity
PV immunohistochemistry results across weeks 1 to 4 are presented in Fig. 4. In the control group, strong PV immunoreactivity was shown in cells scattered in the CA1 and 3 regions (Fig. 4Aa and Ab), and in the DG (4Ac), in addition, weak PV immunoreactivity was detected in the SP in the CA1 and 3 regions (Fig. 4Aa and Ab), and in the GCL in the DG (Fig. 4Ac). In the Scop groups, PV immunoreactivity in the CA1 region was similar to that in the control group at 1 week (Fig. 4Ba and F), thereafter, PV immunoreactivity in the CA1 region was gradually and significantly reduced with time (Fig. 4Ca, Da, Ea and F). In the CA3 region, PV immunoreactivity was not significantly altered at any time after Scop treatment (Fig. 4Bb, Cb, Db, Eb and F). In the DG, PV immunoreactivity was not changed at 1 week after Scop treatment (Fig. 4Bc and F), and significantly reduced from 2 weeks after Scop treatment (Fig. 4Cc, Dc, Ec and F).
Figure 4.
PV immunohistochemistry in the hippocampal subregions of the control (Aa-Ac), 1 wk-(Ba-Bc), 2 wk-(Ca-Cc), 3 wk-(Da-Dc), and 4 wk-(Ea-Ec) Scop groups. In the control group, strong PV immunoreactivity is shown in scattered cells (arrows) in the CA1, CA3 region, and DG, and weak PV immunoreactivity is detected in cells in the SP of the CA1 and CA3 regions, and in the GCL of the DG. PV immunoreactivity is gradually and significantly decreased in the CA1 region and the DG, not in the CA3 region, from 2 weeks after Scop treatment. Scar bar, 50 µm. (F) ROD of PV immunoreactive structures in the control and Scop groups (n=7 per group; *P<0.05 vs. the control group; †P<0.05 vs. the pre-time point Scop group). The bars indicate the SEM. PV, parvalbumin; wk, week; Scop, scopolamine; DG, dentate gyrus; GCL, granule cell layer; ML, molecular layer; PL, polymorphic layer; SO, stratum oriens; SP, stratum pyramidale; SR, stratum radiatum; ROD, relative optical density.
Discussion
In the present study, we investigated long-term changes of spatial and short-term memory, and CBPs expressions in the hippocampal subregions for 4 weeks after systemic Scop treatment in mice and found that significant spatial and short-term memory impairments were caused at 1 week post-treatment and maintained until 4 weeks post-treatment using the MWMT and PAT. These results were consistent with a previous study that showed that long-term Scop treatment induced spatial memory deficits at the beginning of 1 week after Scop injection (21). Acetylcholine has been implicated in memory, for instance, hippocampal-dependent memory (2). It has been well established that systemic Scop injection produces learning and memory impairments via dysregulation of cholinergic mechanisms in the hippocampus (2). For modulation of memory impairments, on the other hand, it has been reported that intraseptal muscarinic agonists alleviate systemic Scop induced-memory impairments (22). In addition, the septo-hippocampal GABAergic fibers are more excited by exogenous muscarine (4), and excitation of septo-hippocampal GABA neurons produces a powerful disinhibition of pyramidal neurons and promotes long-term potentiation in the hippocampus via hippocampal interneurons (4,22). Based on these reports, it is likely that modulation of muscarinic receptors could affect Scop-induced memory impairments through hippocampal interneurons. We, therefore, examined alterations of CB, CR, and PV immunoreactivities in the mouse hippocampus to investigate the link between cognitive functions and hippocampal interneurons containing CBPs after long-term systemic Scop treatment.
In the present study, CB immunoreactivity was significantly decreased in all hippocampal subregions from 1 week after Scop treatment, and rarely observed from 3 weeks after Scop treatment. We recently reported that muscarinic acetylcholine receptor M1 (M1R) was expressed in neurons of the SP and granular cell layer, the expression pattern was similar to that of CB in the CA1 area and DG, and Scop treatment significantly reduced M1R immunoreactivity in the hippocampus (23). On the other hand, a previous study reported that decreased CB immunoreactivity in the hippocampal CA1 region and the DG was directly correlated with decreased recognition memory performance in aged mice (24). Therefore, we suggest that CB immunoreactivity is progressively reduced from 1 week and decreased to the lowest immunoreactivity at least 3 weeks after systemic Scop treatment, although impaired spatial and short-term memory functions are induced from 1 week after systemic Scop treatment.
We found, in this study, that CR and PV immunoreactivities were also severely decreased in the CA1 region and in the DG from at least 3 weeks after Scop treatment; in the CA3 region, the immunoreactivities were not significantly altered until 4 weeks after Scop treatment. Pascual et al reported that CR- and PV-immunoreactive interneurons located in the CA1-3 region selectively received GABAergic axons originated from the medial septum in mice (5). In addition, it was reported that CBPs containing interneurons in the hippocampus were involved in inhibition of GABAergic septo-hippocampal neurons, which were the source of disinhibition of pyramidal neurons in the hippocampus in the mouse, rat and monkey (5–7). Furthermore, some researchers reported that about 50% of all muscarinic cholinoceptive nonpyramidal neurons contained PV, and about 90% of the PV containing neurons expressed mAChR in rats (25,26). Those previous findings as well as our present results suggest that markedly decreased CR and PV immunoreactivities in the CA1 region and in the DG of the mouse might be related to Scop-induced memory impairments. However, it needs to study what maintained CR and PV immunoreactivity in the CA3 region during chronic Scop treatment is related to in memory after or during Scop treatment.
Finally, in the present study, we found that PV immunoreactivity was not changed in all hippocampal subregions 1 week after Scop treatment, not like CB and CR immunoreactivities that were decreased from 2 weeks after Scop treatment. To the best of our knowledge, it is likely that PV containing neurons are relatively resistant to short-term (1 week) Scop treatment than CB and CR containing neurons in the hippocampus.
In conclusion, this study identified that CBPs immunoreactivities in the mouse hippocampus were differently decreased during period of systemic Scop treatment, and suggests that Scop-induced CBPs changes or reductions might be differently related according to types of CBPs to spatial and short-term memory deficits.
Acknowledgements
This study was supported by the Bio-Synergy Research Project (NRF-2015M3A9C4076322) of the Ministry of Science, ICT and Future Planning through the National Research Foundation, and by a Priority Research Centers Program grant (NRF-2009-0093812) through the National Research Foundation of Korea funded by the Ministry of Science, ICT and Future Planning.
References
- 1.Van der Zee EA, Luiten PG. Muscarinic acetylcholine receptors in the hippocampus, neocortex and amygdala: A review of immunocytochemical localization in relation to learning and memory. Prog Neurobiol. 1999;58:409–471. doi: 10.1016/S0301-0082(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 2.Easton A, Douchamps V, Eacott M, Lever C. A specific role for septohippocampal acetylcholine in memory? Neuropsychologia. 2012;50:3156–3168. doi: 10.1016/j.neuropsychologia.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alreja M, Wu M, Liu W, Atkins JB, Leranth C, Shanabrough M. Muscarinic tone sustains impulse flow in the septohippocampal GABA but not cholinergic pathway: Implications for learning and memory. J Neurosci. 2000;20:8103–8110. doi: 10.1523/JNEUROSCI.20-21-08103.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu M, Shanabrough M, Leranth C, Alreja M. Cholinergic excitation of septohippocampal GABA but not cholinergic neurons: Implications for learning and memory. J Neurosci. 2000;20:3900–3908. doi: 10.1523/JNEUROSCI.20-10-03900.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pascual M, Pérez-Sust P, Soriano E. The GABAergic septohippocampal pathway in control and reeler mice: Target specificity and termination onto Reelin-expressing interneurons. Mol Cell Neurosci. 2004;25:679–691. doi: 10.1016/j.mcn.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Gulyás A, Seress L, Tóth K, Acsády L, Antal M, Freund TF. Septal GABAergic neurons innervate inhibitory interneurons in the hippocampus of the macaque monkey. Neuroscience. 1991;41:381–390. doi: 10.1016/0306-4522(91)90334-K. [DOI] [PubMed] [Google Scholar]
- 7.Acsády L, Halasy K, Freund TF. Calretinin is present in non-pyramidal cells of the rat hippocampus-III. Their inputs from the median raphe and medial septal nuclei. Neuroscience. 1993;52:829–841. doi: 10.1016/0306-4522(93)90532-K. [DOI] [PubMed] [Google Scholar]
- 8.Tóth K, Borhegyi Z, Freund TF. Postsynaptic targets of GABAergic hippocampal neurons in the medial septum-diagonal band of broca complex. J Neurosci. 1993;13:3712–3724. doi: 10.1523/JNEUROSCI.13-09-03712.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattson MP. Calcium and neurodegeneration. Aging Cell. 2007;6:337–350. doi: 10.1111/j.1474-9726.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- 10.Foster TC. Calcium homeostasis and modulation of synaptic plasticity in the aged brain. Aging Cell. 2007;6:319–325. doi: 10.1111/j.1474-9726.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 11.Zhang ZJ, Reynolds GP. A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res. 2002;55:1–10. doi: 10.1016/S0920-9964(01)00188-8. [DOI] [PubMed] [Google Scholar]
- 12.Hwang IK, Nam YS, Chung DW, Lee HS, Yoon YS, Yoo KY, Kang TC, Lee IS, Won MH. Changes in the expression of calbindin D-28k in the gerbil hippocampus following seizure. Neurochem Int. 2004;44:145–152. doi: 10.1016/S0197-0186(03)00132-3. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds GP, Abdul-Monim Z, Neill JC, Zhang ZJ. Calcium binding protein markers of GABA deficits in schizophrenia-postmortem studies and animal models. Neurotox Res. 2004;6:57–61. doi: 10.1007/BF03033297. [DOI] [PubMed] [Google Scholar]
- 14.Holmes GL, Yang Y, Liu Z, Cermak JM, Sarkisian MR, Stafstrom CE, Neill JC, Blusztajn JK. Seizure-induced memory impairment is reduced by choline supplementation before or after status epilepticus. Epilepsy Res. 2002;48:3–13. doi: 10.1016/S0920-1211(01)00321-7. [DOI] [PubMed] [Google Scholar]
- 15.Yan BC, Park JH, Chen BH, Cho JH, Kim IH, Ahn JH, Lee JC, Hwang IK, Cho JH, Lee YL, et al. Long-term administration of scopolamine interferes with nerve cell proliferation, differentiation and migration in adult mouse hippocampal dentate gyrus, but it does not induce cell death. Neural Regen Res. 2014;9:1731–1739. doi: 10.4103/1673-5374.143415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onaolapo OJ, Onaolapo AY, Abiola AA, Lillian EA. Central depressant and nootropic effects of daytime melatonin in mice. Ann Neurosci. 2014;21:90–96. doi: 10.5214/ans.0972.7531.210304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doguc DK, Delibas N, Vural H, Altuntas I, Sutcu R, Sonmez Y. Effects of chronic scopolamine administration on spatial working memory and hippocampal receptors related to learning. Behav Pharmacol. 2012;23:762–770. doi: 10.1097/FBP.0b013e32835a38af. [DOI] [PubMed] [Google Scholar]
- 18.Yoo DY, Kim W, Lee CH, Shin BN, Nam SM, Choi JH, Won MH, Yoon YS, Hwang IK. Melatonin improves D-galactose-induced aging effects on behavior, neurogenesis, and lipid peroxidation in the mouse dentate gyrus via increasing pCREB expression. J Pineal Res. 2012;52:21–28. doi: 10.1111/j.1600-079X.2011.00912.x. [DOI] [PubMed] [Google Scholar]
- 19.Ahn JH, Choi JH, Park JH, Kim IH, Cho JH, Lee JC, Koo HM, Hwangbo G, Yoo KY, Lee CH, et al. Long-term exercise improves memory deficits via restoration of myelin and microvessel damage, and enhancement of neurogenesis in the aged gerbil hippocampus after ischemic stroke. Neurorehabil Neural Repair. 2016;30:894–905. doi: 10.1177/1545968316638444. [DOI] [PubMed] [Google Scholar]
- 20.Bae EJ, Chen BH, Shin BN, Cho JH, Kim IH, Park JH, Lee JC, Tae HJ, Choi SY, Kim JD, et al. Comparison of immunoreactivities of calbindin-D28k, calretinin and parvalbumin in the striatum between young, adult and aged mice, rats and gerbils. Neurochem Res. 2015;40:864–872. doi: 10.1007/s11064-015-1537-x. [DOI] [PubMed] [Google Scholar]
- 21.Park JH, Choi HY, Cho JH, Kim IH, Lee TK, Lee JC, Won MH, Chen BH, Shin BN, Ahn JH, et al. Effects of chronic scopolamine treatment on cognitive impairments and myelin basic protein expression in the mouse hippocampus. J Mol Neurosci. 2016;59:579–589. doi: 10.1007/s12031-016-0780-1. [DOI] [PubMed] [Google Scholar]
- 22.Givens B, Olton DS. Bidirectional modulation of scopolamine-induced working memory impairments by muscarinic activation of the medial septal area. Neurobiol Learn Mem. 1995;63:269–276. doi: 10.1006/nlme.1995.1031. [DOI] [PubMed] [Google Scholar]
- 23.Chen BH, Park JH, Kim DW, Park J, Choi SY, Kim IH, Cho JH, Lee TK, Lee JC, Lee CH, et al. Melatonin improves cognitive deficits via restoration of cholinergic dysfunction in a mouse model of scopolamine-induced amnesia. ACS Chem Neurosci. 2017 Sep 27; doi: 10.1021/acschemneuro.7b00278. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 24.Soontornniyomkij V, Risbrough VB, Young JW, Soontornniyomkij B, Jeste DV, Achim CL. Hippocampal calbindin-1 immunoreactivity correlate of recognition memory performance in aged mice. Neurosci Lett. 2012;516:161–165. doi: 10.1016/j.neulet.2012.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van der Zee EA, Luiten PG. GABAergic neurons of the rat dorsal hippocampus express muscarinic acetylcholine receptors. Brain Res Bull. 1993;32:601–609. doi: 10.1016/0361-9230(93)90161-4. [DOI] [PubMed] [Google Scholar]
- 26.Van der Zee EA, de Jong GI, Strosberg AD, Luiten PG. Parvalbumin-positive neurons in rat dorsal hippocampus contain muscarinic acetylcholine receptors. Brain Res Bull. 1991;27:697–700. doi: 10.1016/0361-9230(91)90048-O. [DOI] [PubMed] [Google Scholar]