Abstract
Background
Children with Down Syndrome (DS) are at increased risk of developing acute leukemia and more prone to acute toxicities. We studied the incidence and severity of chronic health conditions among survivors of childhood leukemia with DS compared to those without DS.
Methods
Chronic health conditions reported by questionnaire were compared between 154 pediatric leukemia survivors with DS and 581 without DS, matched by leukemia, age at diagnosis, race/ethnicity, sex, radiation location and chemotherapy exposure using Cox models to estimate Hazard Ratios (HR) and 95% Confidence Intervals [CI]. Subjects were selected from 7,139 five-year survivors of leukemia in the Childhood Cancer Survivor Study.
Results
Risk of at least one late onset chronic health condition (grade 1–5) was similar in the DS population compared to the non-DS group (HR=1.1[0.7–1.5]). Serious chronic health conditions (grade 3–5) were more common in DS survivors (HR=1.7[1.1–2.6]) as were ≥3 chronic health conditions (grades 1–5) (HR=1.7[1.2–2.4]). The 25-year cumulative incidence of any condition (grades 1–5) was 83% for DS survivors and 69% for non-DS survivors.
Conclusions
Leukemia survivors with DS have comparable therapy-related chronic health conditions as similarly treated survivors without DS with few notable exceptions. They have: 1) increased risk of cataracts, hearing loss, and thyroid dysfunction compared to survivors without DS, but these are known risks in the DS population; 2) decreased risk of second cancers; and 3) increased risk of severe or multiple conditions. Practitioners should be aware of these risks during and after therapy.
Keywords: Down syndrome, Leukemia, Late effects, Chronic conditions, Survivorship
Introduction
Down syndrome (DS), caused by trisomy of chromosome 21, is the most common congenital genetic anomaly occurring in 1 out of approximately 700 to 1000 live births.1, 2 The prevalence of DS is increasing which has been attributed to an increasing incidence of DS and to the fact that more people with DS are living longer with current median life expectancy exceeding50 years.2–4 Children with DS are at higher risk for developing acute lymphoblastic leukemia (ALL) and acute myelogenous leukemia (AML) compared to children without DS,5, 6 and those undergoing cancer therapy are at higher risk for prolonged hospitalizations and acute, treatment-related toxicities, such as infections, mucositis and hyperglycemia.7–12 Children and adults with DS without a history of cancer are also known to have a higher risk of certain chronic medical conditions including cataracts, hearing loss, congenital heart abnormalities, and endocrine issues.13–15 However, there are minimal data regarding the late sequelae of cancer therapy in children with DS and leukemia.
Since children with DS diagnosed with leukemia are at higher risk of acute toxicities and many will survive into adulthood where they are predisposed to chronic conditions, we hypothesized that they may be at increased risk of chronic health conditions compared to children with leukemia without DS. To test this hypothesis, we conducted a matched cohort analysis within the Childhood Cancer Survivor Study (CCSS).
Materials and Method
Patients
The CCSS is a retrospective cohort of 24,368 children who were diagnosed under 21 years of age with a leukemia, lymphoma, central nervous system tumor, Wilms’ tumor, neuroblastoma, sarcoma, or bone tumor between 1970 and 1999 at one of 27 collaborating institutions in the United States and Canada and who survived at least five years after diagnosis. There were 6148 survivors of childhood acute lymphoblastic leukemia (ALL) and 868 survivors of acute myeloid leukemia (AML) within the CCSS cohort. Demographic characteristics and numerous health outcomes were collected from a baseline, self-administered questionnaire through the fourth follow up survey (FU4), for survivors diagnosed 1970–1986, and from an initial baseline questionnaire for survivors diagnosed 1987–1999.
Presence of DS was self-reported (by patient or proxy). The non-DS comparison population was selected by stratified random sampling. A desired ratio of four controls to one case, where possible, were matched for the following characteristics: diagnosis group (type of leukemia: ALL, AML, other), age at diagnosis (to within one year), race (white, non-Hispanic vs. other), cyclophosphamide equivalent dose (CED; <8000 vs ≥ 8000 mg/m2), anthracycline dose (<=250 mg/m2 vs. >250 mg/m2), total body irradiation (TBI[yes/no], for both ALL and AML) and cranial/spinal/testicular [yes/no] radiation (non-AML only). For 130 of the DS, four matching non-DS were found. Fewer than four matches were found for the remaining 24: 17 DS with three non-DS, three DS with two non-DS, and four DS with one non-DS. There were three patients with DS who did not have a non-DS match using these criteria and they were excluded from the analysis. Medical record abstraction of treatment-related factors, including chemotherapy information (i.e., whether chemotherapy had been given [yes/no], method of administration, and cumulative dose), occurred at each local institution. All participants provided informed consent and the study procedures were approved by the regulatory boards at all participating institutions. Vital status and cause of death was ascertained as of December 31, 2013 via linkage to the U.S. National Death Index.
Health Condition Outcomes
The severity grading of chronic health conditions was based on the Common Terminology Criteria for Adverse Events (CTCAE, version 4.03) and included mild (grade 1), moderate (grade 2), severe or disabling (grade 3), life-threatening (grade 4), or fatal (grade 5) events.16 A total of 61 different health conditions were scored across all questionnaires. If there was not enough information to distinguish between grades, the lower score was recorded. Onset of a chronic condition was determined based on self-report of age at first occurrence. Equivalent onset dates of the conditions were calculated using the mid-point of the year of the reported age.
Conditions were further summarized across systems (cardiac, endocrine, etc.) using the maximum grade and earliest reported age. Summaries did not include problems with learning/ memory or ovarian failure/testicular dysfunction as these would be expected in patients with DS. Health conditions before the cancer diagnosis or within five years of diagnosis were quantified, but not included in the comparative models.
Statistical analysis
Frequencies for chronic conditions, categorized by time period, were calculated for DS and non-DS leukemia survivors. We used Cox proportional hazard models to calculate hazard ratios (HR) and 95% confidence intervals (CI) of late chronic medical conditions, comparing DS leukemia survivors to non-DS leukemia survivors. Survivors entered the analysis at five years post-diagnosis and were followed until the earliest chronic condition, death or last follow-up. Separate univariate models were used for individual conditions and summaries. Hazard ratios were not estimable for all chronic conditions, due to the low number of events after cohort entry. In additional analyses among only those with AML or ALL, interactions were tested between group status and diagnosis group (AML/ALL) and separate HR estimates for the diagnosis groups were generated for those outcomes indicating a significant interaction at a level of 0.10 or less.
Cumulative incidence curves for any grade 1–5 condition and any grade 3–5 condition among patients with DS or without DS were estimated, with death from causes other than a chronic condition as a competing risk.17 Cumulative incidence was also evaluated for individual health conditions with an adequate number of events, e.g. hypothyroidism, and by type of leukemia (ALL, AML). All cumulative incidence curves started at the prevalence at cohort entry (five years from diagnosis) to illustrate the full burden of conditions in this population and were truncated at 25 years due to the low numbers of survivors still at risk after that point. Survival estimates were generated for DS and non-DS using the Kaplan-Meier estimator, with comparisons between DS and non-DS based on the logrank test.
All analyses, including reported percentages, were weighted to account for under-sampling of ALL survivors in the latter recruitment era (1987–1999), with a weight of 1.21 for ALL age 0 or 11–20 years at diagnosis, and a weight of 3.63 for those age 1–10 years. AML subjects had a weight of 1. Frequency counts represent unweighted numbers.
Results
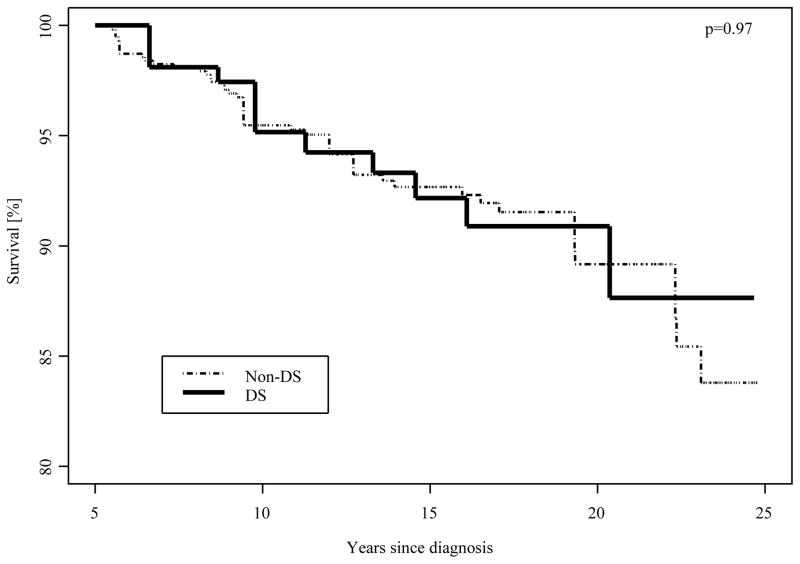
There were 154 survivors of leukemia with DS, and 581 leukemia survivors without DS matched for comparison (Table 1). Age at last follow-up was evenly distributed with the majority of survivors between 10–39 years old. Median age at last follow-up was 20 (range 10–48) years for DS and 22 (range 7– 49) years for non-DS survivors. Sixty-five (59.3% weighted) of the survivors with DS had ALL and 81 (37.1% weighted) had AML. The overall survival beyond five years from diagnosis was similar for survivors with and without DS (88% vs 84% at 25 years post-diagnosis, p=0.97, Figure 1); overall survival by leukemia subtype (AML, 84% vs ALL, 85%) was also similar for children with and without DS (p=0.39 and 0.80, respectively, ).
Table 1.
Demographic and treatment characteristics of CCSS leukemia survivors with DS and non-DS matched comparison population
| DS | non-DS | ||||
|---|---|---|---|---|---|
| N | %** | N | %** | ||
| Total | 154 | 100.0 | 581 | 100.0 | |
| Age at last follow-up | <10 | 0 | 0.0 | 4 | 0.5 |
| 10–19 | 65 | 47.8 | 178 | 36.5 | |
| 20–29 | 62 | 39.4 | 297 | 49.3 | |
| 30–39 | 21 | 10.0 | 89 | 12.0 | |
| 40+ | 6 | 2.7 | 13 | 1.7 | |
| Gender* | Female | 63 | 43.7 | 238 | 41.9 |
| Male | 91 | 56.3 | 343 | 58.1 | |
| Years of follow-up since diagnosis | 5–14 | 47 | 37.6 | 142 | 30.1 |
| 15–19 | 39 | 24.1 | 153 | 28.0 | |
| 20–24 | 48 | 29.2 | 171 | 26.9 | |
| 25–29 | 14 | 6.4 | 77 | 10.2 | |
| 30–34 | 5 | 2.3 | 30 | 3.8 | |
| 35+ | 1 | 0.5 | 8 | 1.0 | |
| Race/ethnicity* | White non-Hispanic | 123 | 80.8 | 470 | 80.6 |
| Other race | 31 | 19.2 | 111 | 19.4 | |
| Ethnicity | Hispanic | 15 | 10.6 | 65 | 11.1 |
| Non-Hispanic | 139 | 89.4 | 516 | 88.9 | |
| Race | American Indian / Alaska Native | 1 | 0.5 | 5 | 1.0 |
| Asian or Pacific Islander | 3 | 2.6 | 4 | 1.2 | |
| Black | 11 | 5.1 | 27 | 4.6 | |
| Other | 10 | 5.9 | 43 | 7.6 | |
| Unknown | 2 | 2.1 | 3 | 0.7 | |
| White | 127 | 83.8 | 499 | 85.0 | |
| Diagnosis* | Acute lymphoblastic leukemia | 65 | 59.3 | 254 | 58.3 |
| Acute myeloid leukemia | 81 | 37.1 | 311 | 39.6 | |
| Other leukemia | 8 | 3.7 | 16 | 2.0 | |
| Age at cancer diagnosis* | <1 | 15 | 6.9 | 75 | 9.6 |
| 1–4 | 103 | 62.8 | 382 | 64.4 | |
| 5–16 | 29 | 26.7 | 102 | 22.7 | |
| ≥17 | 7 | 3.6 | 22 | 3.2 | |
| Status | Alive | 142 | 91.9 | 541 | 93.1 |
| Deceased | 12 | 8.1 | 40 | 6.9 | |
| Year of primary cancer diagnosis | 1970–73 | 2 | 0.9 | 26 | 3.3 |
| 1974–77 | 5 | 2.3 | 34 | 4.3 | |
| 1978–81 | 15 | 6.9 | 76 | 9.7 | |
| 1982–86 | 32 | 14.6 | 149 | 19.0 | |
| 1987–90 | 28 | 21.5 | 77 | 16.6 | |
| 1991–94 | 22 | 15.0 | 83 | 16.8 | |
| 1995–99 | 50 | 38.8 | 136 | 30.3 | |
| Anthracyclines dose* | <=250 mg/m2 | 103 | 75.8 | 400 | 78.9 |
| >250 mg/m2 | 43 | 24.2 | 151 | 21.1 | |
| CED ≥8000 mg/m2* | Yes | 8 | 4.0 | 30 | 4.2 |
| No | 136 | 96.0 | 512 | 95.8 | |
| Any Radiation | Yes | 25 | 16.8 | 144 | 22.9 |
| No | 123 | 83.2 | 414 | 77.1 | |
| Radiation location* | TBI | 1 | 0.5 | 4 | 0.9 |
| Craniospinal/Testicular (noTBI) | 22 | 15.4 | 136 | 21.4 | |
| Other location (no TBI) | 2 | 0.9 | 4 | 0.6 | |
| No Radiation | 123 | 83.2 | 414 | 77.1 | |
| Proxy | Yes | 130 | 84.4 | 174 | 29.9 |
| No | 24 | 15.6 | 407 | 70.1 | |
indicates used for matching; For radiation location all leukemia survivors were matched on TBI, only ALL survivors were matched on craniospinal.
DS: 7 died of chronic conditions (1 lung cancer, 3 respiratory, 2 cardiomyopathy, 1 stroke), 4 of recurrence of leukemia, 1 other cause
Percentages reflect weighting used for ALL diagnosed 1987–1999, with weight=1.21 for those age <1 or 11–20 at diagnosis, and weight=3.63 for those age 1–10 at diagnosis. Radiation, anthracyclines and CED presented only among those with medical records abstraction.
Figure 1.
Kaplan-Meier survival among 5-year survivors of leukemia with and without DS
Of 154 survivors with DS, 123 reported a chronic health condition (grade 1–5). The majority (n= 99, 63.7%) reported onset prior to diagnosis or between diagnosis and five years after diagnosis, with only 24 (15.9%) reporting onset of new health condition more than five years after diagnosis (Table 2). Fifty survivors of leukemia with DS reported severe conditions (grade 3–5), with 28 (16.6%) reporting onset prior to five years after diagnosis and 22 (13.8%) more than five years after diagnosis.
Table 2.
Prevalence and timing of health conditions for CCSS leukemia survivors and Hazard Ratios (HR) for chronic conditions in DS vs. non-DS
| Group | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DS | non-DS | |||||||||||||
| Chronic Health Conditions (>5 years from diagnosis) | No/None | <5 year from diagnosis | ≥5 years from diagnosis | No/None | <5 year from diagnosis | ≥5 years from diagnosis | ||||||||
| HR (95% CI) | P | N | % | N | % | N | % | N | % | N | % | N | % | |
| Any grade 1 to 5 | 1.1 ( 0.7– 1.5) | 0.78 | 31 | 20.4 | 99 | 63.7 | 24 | 15.9 | 229 | 38.8 | 189 | 33.9 | 163 | 27.3 |
| Any grade 3–5 | 1.7 (1.1– 2.6) | 0.02 | 104 | 69.6 | 28 | 16.6 | 22 | 13.8 | 475 | 82.5 | 48 | 7.3 | 58 | 10.2 |
| ≥2, grades 1–5 | 1.2 (0.9– 1.7) | 0.24 | 56 | 37.9 | 65 | 42.0 | 33 | 20.1 | 359 | 60.2 | 85 | 13.7 | 137 | 16.1 |
| ≥3, grades 1–5 | 1.7 (1.2– 2.4) | 0.003 | 84 | 56.8 | 37 | 23.2 | 33 | 20.0 | 444 | 75.5 | 51 | 7.9 | 86 | 16.5 |
| Major joint Replacement | 4.2 (1.0–18.5) | 0.06 | 153 | 98.3 | 0 | 0.0 | 1 | 1.7 | 576 | 99.3 | 2 | 0.3 | 3 | 0.4 |
| Congestive heart failure | 1.8 (0.7– 4.8) | 0.23 | 142 | 94.5 | 6 | 2.7 | 6 | 2.7 | 560 | 97.2 | 9 | 1.1 | 12 | 1.6 |
| Second malignant neoplasm | 0.1 (0.0– 0.9) | 0.04 | 153 | 99.5 | 0 | 0.0 | 1 | 0.5 | 554 | 95.6 | 1 | 0.1 | 26 | 4.3 |
| Coronary artery disease | 1.2 (0.1–11.4) | 0.87 | 152 | 99.1 | 1 | 0.5 | 1 | 0.5 | 577 | 99.5 | 1 | 0.1 | 3 | 0.4 |
| Cerebrovascular accident | 0.6 (0.1– 2.7) | 0.52 | 147 | 95.5 | 5 | 3.6 | 2 | 0.9 | 570 | 97.2 | 7 | 1.2 | 4 | 1.5 |
| Hearing loss | 3.4 (1.9– 6.0) | <0.0001 | 115 | 73.7 | 22 | 16.13 | 17 | 10.2 | 531 | 91.6 | 31 | 4.6 | 19 | 3.8 |
| Blindness | 1.8 (0.7– 4.6) | 0.21 | 145 | 94.7 | 5 | 2.3 | 4 | 3.0 | 565 | 96.6 | 10 | 1.6 | 6 | 1.8 |
| Cataracts | 3.8 (2.2– 6.7) | <0.0001 | 138 | 89.0 | 0 | 0.0 | 16 | 11.0 | 545 | 94.0 | 17 | 2.9 | 19 | 3.1 |
| Any endocrine effects* | 1.9 (1.4– 2.6) | 0.0001 | 105 | 65.4 | 14 | 10.0 | 35 | 24.6 | 467 | 79.6 | 30 | 4.6 | 84 | 15.8 |
| Hypothyroidism | 3.6 ( 2.4– 5.5) | <0.0001 | 117 | 73.3 | 10 | 8.2 | 27 | 18.5 | 543 | 92.7 | 9 | 1.1 | 29 | 6.1 |
| Diabetes | 1.5 ( 0.7– 3.3) | 0.30 | 148 | 96.0 | 0 | 0.0 | 6 | 4.0 | 561 | 96.1 | 7 | 0.9 | 13 | 3.0 |
| Hyperthyroid | 3.8 ( 1.0–14.2) | 0.05 | 150 | 98.2 | 0 | 0.0 | 4 | 1.8 | 575 | 98.9 | 1 | 0.5 | 5 | 0.9 |
| Any respiratory effects** | 0.7 ( 0.4– 1.2) | 0.16 | 124 | 77.7 | 22 | 16.1 | 8 | 6.2 | 484 | 83.6 | 37 | 5.7 | 60 | 10.7 |
composite of hyperthyroid, hypothyroid, thyroid nodules, diabetes, growth hormone deficiency, osteoporosis and other hormonal conditions
composite of chronic cough, emphysema, lung fibrosis and other respiratory conditions
Each row represents a separate Cox regression model; HR=Hazard Ratio, CI=Confidence Interval. HR, CI and percentages reflect weighting used for ALL diagnosed 1987–1999, with weight=1.21 for those age 0 or 11–20 at diagnosis, and weight=3.63 for those age 1–10 at diagnosis
HRs for any chronic condition (grade 1–5) reported at least five years after diagnosis identified no increased risk for DS compared to non-DS survivors (HR=1.1; 95% CI 0.7–1.5, p=0.78, Table 2). The risk for severe, life-threatening, disabling and fatal late chronic health conditions (grade 3–5) was higher for leukemia survivors with DS (HR=1.7; 95% CI 1.1–2.6, p=0.02). The risk for more than one health condition was similar for the two groups (HR=1.2; 95% CI 0.9–1.7, p=0.24); however, the risk for three or more chronic conditions was greater in patients with DS (HR=1.7; 95% CI 1.2–2.4, p=0.003). HRs comparing DS to non-DS survivors on 14 selected conditions/organ systems are provided in Table 2. DS leukemia survivors were at significantly higher risk for hearing loss (HR=3.4; 95% CI 1.9–6.0, p<0.0001), endocrine effects (HR=1.9; 95% CI 1.4–2.6, p=0.0001) and cataracts (HR=3.8; 95% CI 2.2–6.7, p<0.0001), but lower risk for second malignant neoplasms (HR=0.1; 95% CI 0.0–0.9, p=0.04) compared to leukemia survivors without DS. Of note, there was no significant difference in the risk of congestive heart disease in survivors of leukemia with and without DS (HR = 1.8; 95% CI 0.7– 4.8, p=0.23). Additional models tested interactions with diagnosis group (ALL vs. AML). Among AML patients, the risk of multiple conditions was significantly higher for DS vs. non-DS (HR=2.4; 95% CI [1.4–4.0, p=0.002 for 2+ conditions, HR=3.3; 95% CI 1.9–5.7, p<0.0001 for 3+ conditions), while for survivors of ALL, the risk for these outcomes was similar between those with and without DS (HR=0.9; 95% CI [0.6–1.3], p=0.53 for 2+, HR=1.2; 95% CI [0.8–1.9], p=0.37 for 3+).
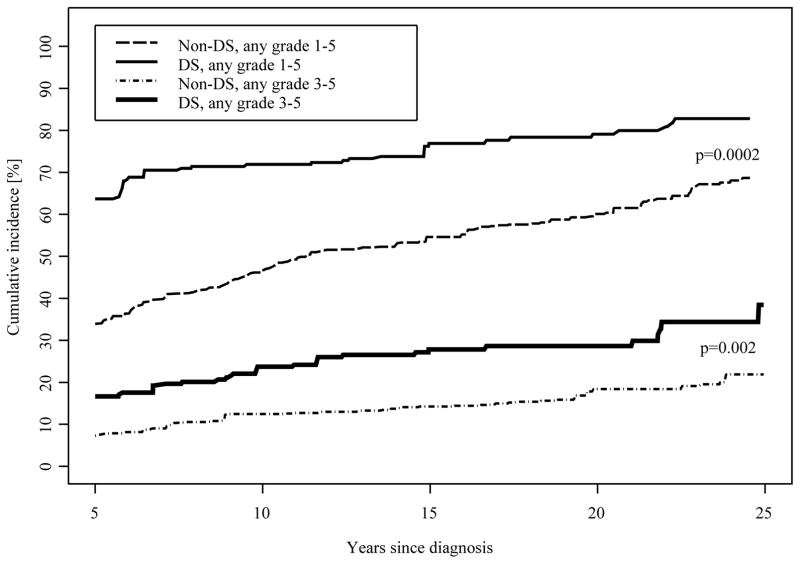
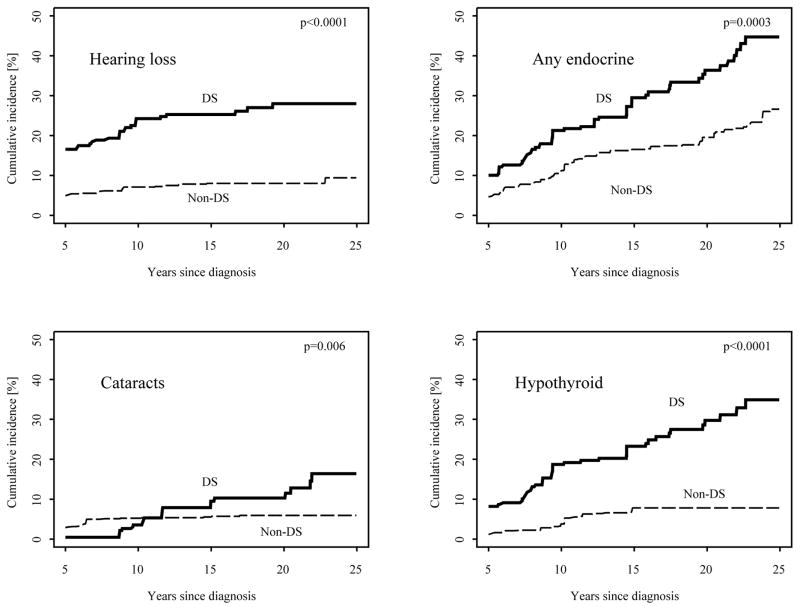
By 25 years after cancer diagnosis, the cumulative incidence of having developed at least one chronic health condition was 83% (95% CI 77%–88%) for DS survivors and 69% (95% CI 65%–74%) for survivors of leukemia without DS matched for specific treatment exposures (Figure 2). These differences were largely attributable to the fact that survivors with DS had an increased prevalence at five years (64% vs. 34%) compared to those without DS. The cumulative incidence of any grade 3–5 chronic condition by 25 years after diagnosis was 35% (95% CI 27%–43%) for survivors of leukemia with DS and 22% (95% CI 18%–25%) for survivors without DS. The cumulative incidence of any chronic condition as well as for any grade 3–5 chronic condition were similar when stratified by leukemia subtype (Supplementary Figure). Figure 3 shows the cumulative incidence of four selected conditions for which cumulative incidence was higher in survivors of leukemia with DS compared to matched non-DS survivors. Of note, these data suggest most hearing loss occurs within 10 years of diagnosis whereas new occurrences of cataracts and endocrinopathies continue over time. Secondary malignant neoplasms (SMN) were less common among survivors with DS than survivors without DS, with a cumulative incidence of 6% (95% CI 4%–9%) among survivors without DS compared to no cases among survivors with DS at 25 years after diagnosis.
Figure 2.
Cumulative incidence of chronic conditions in DS and non-DS leukemia survivors, by grade
Figure 3.
Cumulative incidence of specific chronic conditions (any grade) in DS and non-DS leukemia survivors. ‘Any endocrine’ is a composite of hyperthyroid, hypothyroid, thyroid nodules, diabetes, growth hormone deficiency, osteoporosis and other hormonal conditions
Discussion
In this study, we found that survivors of childhood leukemia with and without DS had a similar overall risk of chronic conditions (grade 1–5) occurring more than five years after cancer diagnosis. However, survivors with DS were at higher risk of developing more severe (grade 3–5) late chronic conditions, including cataracts, hearing loss and endocrinopathies, but at lower risk for developing SMN.
Five-year overall survival for patients with DS and leukemia varies by type of leukemia. Children with DS and AML fare better than children without DS diagnosed with similar AML subtypes (~80% vs 35%), yet children with DS and ALL fare worse compared to children without DS diagnosed with ALL (~70% vs ~90%).18, 19,20–23 AML in DS patients, termed Myeloid Leukemia of DS (ML-DS), is specific to trisomy 21 and is clinically and genetically distinct from AML occurring in non-DS patients. Improved survival in DS patients with AML may be due to an increased sensitivity of their leukemia cells to commonly used AML agents like cytosine arabinoside.24–26 Whether this increased sensitivity could impact other cells and influence risk of late effects is unknown. Children with DS and ALL have fewer favorable biologic features, which presumably contributes to their lower overall survival.12, 20 Children with DS and ALL may require additional therapy to eradicate their disease and this could increase their risk for late effects. It is also well established that children with DS diagnosed with leukemia do experience higher rates of certain acute toxicities including mucositis, metabolic disturbances, sepsis and pulmonary complications.27, 28 The increased acute toxicities could suggest this population may be at higher risk of late effects as well. As survival continues to improve for children with DS diagnosed with leukemia utilizing risk-based therapeutic strategies, understanding the long-term risks becomes more paramount.
Previous reports on late effects in leukemia survivors with DS are sparse. A recent report from the Children’s Oncology Group showed that 77% of AML survivors with DS reported at least 1 chronic medical condition compared to 50% of AML patients without DS.29 A report evaluating late cardiomyopathy in AML patients with and without DS showed no difference in the incidence of this condition.30 We also identified that children with DS and leukemia do not seem at higher risk for late cardiac conditions than those without DS despite of the known increased incidence of cardiovascular malformations. Another study analyzing the occurrence of clinical cardiotoxicity after anthracyclines for patients treated on Pediatric Oncology Group protocols found DS as a risk factor in multivariate analysis.31 It is possible in our study that children with DS and underlying cardiac disease may not have survived the required five years to be eligible to participate. Other reports of leukemia survivors with DS found that treatment worsened verbal and adaptive function compared to children with DS who did not have leukemia.32 We did not examine problems with learning and memory as these are established deficits in individuals with DS.
We know that people with DS without a history of cancer experience higher rates of hospitalizations and higher rates of many of the chronic conditions identified in our study. 33, 34 Hypothyroidism can occur in up to 40% of adults with DS.35 Mild to severe hearing loss can be seen in nearly 50% of adults with DS.36 Cataracts are also common in adults with DS.37 Thus, our findings may simply reflect the natural history of DS. Nonetheless, we cannot exclude the possibility that treatment for childhood leukemia might exacerbate or increase the risk for some of these conditions.38, 39 Hypotheses of accelerated aging attributable to telomere shortening or cellular senescence have been posited.40 Regardless, clinicians caring for leukemia survivors with DS should be aware of these medical risks and take them in consideration when establishing care and follow-up plans.
Children with DS are prone to medical issues that vary with age. 41 For adolescents and young adults with DS, the American Academy of Pediatrics recommends annual examinations including nutritional assessment (including dietary and iron intake assessments), audiology evaluations, TSH monitoring, ophthalmology evaluation every 2–3 years, appropriate cardiac follow up, neurologic monitoring for myelopathic signs and neurologic dysfunction, and review of co-morbid issues including obesity and sleep apnea. Young people with DS who have survived childhood leukemia should, at a minimum, follow these follow up recommendations.
The low risk of subsequent cancers noted in this study is of interest. Only 1 case of SMN was noted, namely an adenocarcinoma in a survivor of ALL with DS occurring 29 years from original diagnosis. In the treatment matched cohort without DS, 26 (4.3%) developed SMN. Another large study with good clinical follow-up of ALL survivors found a similar cumulative incidence of 4.2% at 15 years.42 The lower risk for SMNs may reflect the established fact that children and adults with DS are actually at lower risk of solid tumors compared to the general population.43 People with DS are at significantly lower risk of breast, lung and cervical cancer. 44 The lower risk of second cancers in DS survivors may indicate a potential protective effect of one or more chromosome 21-localized genes in reducing the risk of secondary cancers. Studies assessing genetic and environmental modifiers that may influence these risks are warranted. How the low rates of certain solid tumors, like breast cancer, should affect cancer screening among survivors of childhood cancer with have been raised.45
The lack of an available comparison group of individuals with DS without leukemia prevents us from determining whether the chronic conditions are due to DS or the leukemia therapy. The fact that more chronic conditions were present before 5 years from diagnosis suggests that these conditions may be more related to DS than related to the cancer therapy. While all individuals with DS are at risk for vision, hearing and endocrine conditions, survivors of childhood leukemia with DS may be more prone to or have accelerated onset of these medical conditions. While not feasible within the confines of the CCSS cohort, future studies regarding the relative contributions are warranted.
While the small population size and self-reported nature of chronic health conditions are also limitations of this study, to our knowledge, this is the largest study assessing the potential long-term complications in children with DS and leukemia. The higher proportion of DS survivors with proxy reports by parents provides a potential for bias in the results (84% for DS survivors vs 30% by non-DS survivors). However, prior studies suggest parents are more likely to worry more about future health and late effects than patients and over-report events46; thus, the bias would likely over-estimate the risk of chronic conditions in the DS survivors. It is possible that the follow up for survivors of leukemia with DS may be more rigorous than survivors without DS. Again, this would potentially increase the number of chronic conditions reported in the DS population. The use of a matched comparison cohort reduces potential confounding bias based on diagnosis and treatment-specific factors. While the matching of for radiation exposure was based on location of radiation and was different for AML and ALL, the overall exposure to any radiation was slightly higher among non-DS survivors (23% vs. 17%). Thus, the increased risk for multiple conditions in the DS survivors is less likely due to differences in these therapeutic exposures and more likely related to known DS conditions or differences in sensitivity to treatment exposures. Additionally, newer therapy regimens developed in the Children’s Oncology Group take into account the unique clinical and biologic features of leukemia in DS patients in an effort to reduce toxicities; hopefully this will reduce any potential added risk of chronic health conditions due to therapy in this population.
This study demonstrates that survivors of leukemia with DS have an overall similar risk of any late complications, but are at higher risk for specific and more severe conditions. The more common late occurring medical conditions, cataracts, hearing loss, and thyroid disorders, may be solely related to the underlying DS. Regardless, providers caring for this population of patients should be aware of these risks during therapy as they consider treatment options and after therapy as they monitor for late occurring medical conditions.
Supplementary Material
Supplementary Figure: Cumulative incidence of chronic conditions by type of leukemia in patients with DS
Acknowledgments
This work was supported by the National Cancer Institute (CA55727, GTA, Principal Investigator), Alex Lemonade Stand Foundation (SR), and Swim Across America (REG). Support to St. Jude Children’s Research Hospital also provided by the Cancer Center Support (CORE) grant (CA21765, C. Roberts, Principal Investigator), the American Lebanese-Syrian Associated Charities (ALSAC). We acknowledge Linda Li for her help in initiating this study.
Footnotes
Author Contributions and Conflicts of Interest:
Robert E. Goldsby - designed research, performed research, analyzed data, and wrote the paper. No COI.
Kayla L. Stratton - designed research, performed research, analyzed data, and wrote the paper. No COI.
Shannon Raber- designed research, performed research, and wrote the paper. No COI.
Arthur Ablin - designed research, analyzed data, and wrote the paper. No COI.
Louise C. Strong - designed research, analyzed data, and wrote the paper. No COI.
Kevin Oeffinger - designed research, analyzed data, and wrote the paper. No COI.
Charles A. Sklar - designed research, analyzed data, and wrote the paper. Honoraria from Sorono.
Gregory T. Armstrong - designed research, performed research, analyzed data, and wrote the paper. No COI.
Leslie L. Robison - designed research, analyzed data, and wrote the paper. No COI.
Smita Bhatia- designed research, analyzed data, and wrote the paper. No COI.
Wendy M. Leisenring - designed research, performed research, analyzed data, and wrote the paper. No COI.
References
- 1.Parker SE, Mai CT, Canfield MA, et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol. 2010;88:1008–1016. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- 2.Shin M, Besser LM, Kucik JE, Lu C, Siffel C, Correa A. Prevalence of Down syndrome among children and adolescents in 10 regions of the United States. Pediatrics. 2009;124:1565–1571. doi: 10.1542/peds.2009-0745. [DOI] [PubMed] [Google Scholar]
- 3.Irving C, Basu A, Richmond S, Burn J, Wren C. Twenty-year trends in prevalence and survival of Down syndrome. Eur J Hum Genet. 2008;16:1336–1340. doi: 10.1038/ejhg.2008.122. [DOI] [PubMed] [Google Scholar]
- 4.Yang Q, Rasmussen SA, Friedman JM. Mortality associated with Down’s syndrome in the USA from 1983 to 1997: a population-based study. Lancet. 2002;359:1019–1025. doi: 10.1016/s0140-6736(02)08092-3. [DOI] [PubMed] [Google Scholar]
- 5.Goldacre MJ, Wotton CJ, Seagroatt V, Yeates D. Cancers and immune related diseases associated with Down’s syndrome: a record linkage study. Arch Dis Child. 2004;89:1014–1017. doi: 10.1136/adc.2003.046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasle H, Clemmensen IH, Mikkelsen M. Risks of leukaemia and solid tumours in individuals with Down’s syndrome. Lancet. 2000;355:165–169. doi: 10.1016/S0140-6736(99)05264-2. [DOI] [PubMed] [Google Scholar]
- 7.Chessells JM, Harrison G, Richards SM, et al. Down’s syndrome and acute lymphoblastic leukaemia: clinical features and response to treatment. Arch Dis Child. 2001;85:321–325. doi: 10.1136/adc.85.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dordelmann M, Schrappe M, Reiter A, et al. Down’s syndrome in childhood acute lymphoblastic leukemia: clinical characteristics and treatment outcome in four consecutive BFM trials. Berlin-Frankfurt-Munster Group. Leukemia. 1998;12:645–651. doi: 10.1038/sj.leu.2400989. [DOI] [PubMed] [Google Scholar]
- 9.Kalwinsky DK, Raimondi SC, Bunin NJ, et al. Clinical and biological characteristics of acute lymphocytic leukemia in children with Down syndrome. Am J Med Genet Suppl. 1990;7:267–271. doi: 10.1002/ajmg.1320370753. [DOI] [PubMed] [Google Scholar]
- 10.Levitt GA, Stiller CA, Chessells JM. Prognosis of Down’s syndrome with acute leukaemia. Arch Dis Child. 1990;65:212–216. doi: 10.1136/adc.65.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ragab AH, Abdel-Mageed A, Shuster JJ, et al. Clinical characteristics and treatment outcome of children with acute lymphocytic leukemia and Down’s syndrome. A Pediatric Oncology Group study. Cancer. 1991;67:1057–1063. doi: 10.1002/1097-0142(19910215)67:4<1057::aid-cncr2820670432>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 12.Bassal M, La MK, Whitlock JA, et al. Lymphoblast biology and outcome among children with Down syndrome and ALL treated on CCG-1952. Pediatr Blood Cancer. 2005;44:21–28. doi: 10.1002/pbc.20193. [DOI] [PubMed] [Google Scholar]
- 13.American Academy of Pediatrics. Health supervision for children with Down syndrome. Pediatrics. 2001;107:442–449. doi: 10.1542/peds.107.2.442. [DOI] [PubMed] [Google Scholar]
- 14.Cohen WI, Nadel L, Madnick ME. Down syndrome : visions for the 21st century. New York: Wiley-Liss; 2002. [Google Scholar]
- 15.Henderson A, Lynch SA, Wilkinson S, Hunter M. Adults with Down’s syndrome: the prevalence of complications and health care in the community. Br J Gen Pract. 2007;57:50–55. [PMC free article] [PubMed] [Google Scholar]
- 16.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 17.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 18.Craze JL, Harrison G, Wheatley K, Hann IM, Chessells JM. Improved outcome of acute myeloid leukaemia in Down’s syndrome. Arch Dis Child. 1999;81:32–37. doi: 10.1136/adc.81.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravindranath Y, Abella E, Krischer JP, et al. Acute myeloid leukemia (AML) in Down’s syndrome is highly responsive to chemotherapy: experience on Pediatric Oncology Group AML Study 8498. Blood. 1992;80:2210–2214. [PubMed] [Google Scholar]
- 20.Buitenkamp TD, Izraeli S, Zimmermann M, et al. Acute lymphoblastic leukemia in children with Down syndrome: a retrospective analysis from the Ponte di Legno study group. Blood. 2014;123:70–77. doi: 10.1182/blood-2013-06-509463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitlock JA, Sather HN, Gaynon P, et al. Clinical characteristics and outcome of children with Down syndrome and acute lymphoblastic leukemia: a Children’s Cancer Group study. Blood. 2005;106:4043–4049. doi: 10.1182/blood-2003-10-3446. [DOI] [PubMed] [Google Scholar]
- 22.Caldwell JT, Ge Y, Taub JW. Prognosis and management of acute myeloid leukemia in patients with Down syndrome. Expert Rev Hematol. 2014;7:831–840. doi: 10.1586/17474086.2014.959923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patrick K, Wade R, Goulden N, et al. Outcome of Down syndrome associated acute lymphoblastic leukaemia treated on a contemporary protocol. Br J Haematol. 2014;165:552–555. doi: 10.1111/bjh.12739. [DOI] [PubMed] [Google Scholar]
- 24.Taub JW, Ge Y. Down syndrome, drug metabolism and chromosome 21. Pediatr Blood Cancer. 2005;44:33–39. doi: 10.1002/pbc.20092. [DOI] [PubMed] [Google Scholar]
- 25.Taub JW, Huang X, Matherly LH, et al. Expression of chromosome 21-localized genes in acute myeloid leukemia: differences between Down syndrome and non-Down syndrome blast cells and relationship to in vitro sensitivity to cytosine arabinoside and daunorubicin. Blood. 1999;94:1393–1400. [PubMed] [Google Scholar]
- 26.Taub JW, Matherly LH, Stout ML, Buck SA, Gurney JG, Ravindranath Y. Enhanced metabolism of 1-beta-D-arabinofuranosylcytosine in Down syndrome cells: a contributing factor to the superior event free survival of Down syndrome children with acute myeloid leukemia. Blood. 1996;87:3395–3403. [PubMed] [Google Scholar]
- 27.Gamis AS, Woods WG, Alonzo TA, et al. Increased age at diagnosis has a significantly negative effect on outcome in children with Down syndrome and acute myeloid leukemia: a report from the Children’s Cancer Group Study 2891. J Clin Oncol. 2003;21:3415–3422. doi: 10.1200/JCO.2003.08.060. [DOI] [PubMed] [Google Scholar]
- 28.O’Brien MM, Taub JW, Chang MN, et al. Cardiomyopathy in children with Down syndrome treated for acute myeloid leukemia: a report from the Children’s Oncology Group Study POG 9421. J Clin Oncol. 2008;26:414–420. doi: 10.1200/JCO.2007.13.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schultz KA, Chen L, Kunin-Batson A, et al. Health-related Quality of Life (HR-QOL) and Chronic Health Conditions in Survivors of Childhood Acute Myeloid Leukemia (AML) with Down Syndrome (DS): A Report From the Children’s Oncology Group. J Pediatr Hematol Oncol. 2017;39:20–25. doi: 10.1097/MPH.0000000000000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Creutzig U, Diekamp S, Zimmermann M, Reinhardt D. Longitudinal evaluation of early and late anthracycline cardiotoxicity in children with AML. Pediatr Blood Cancer. 2007;48:651–662. doi: 10.1002/pbc.21105. [DOI] [PubMed] [Google Scholar]
- 31.Krischer JP, Epstein S, Cuthbertson DD, Goorin AM, Epstein ML, Lipshultz SE. Clinical cardiotoxicity following anthracycline treatment for childhood cancer: the Pediatric Oncology Group experience. J Clin Oncol. 1997;15:1544–1552. doi: 10.1200/JCO.1997.15.4.1544. [DOI] [PubMed] [Google Scholar]
- 32.Roncadin C, Hitzler J, Downie A, et al. Neuropsychological late effects of treatment for acute leukemia in children with Down syndrome. Pediatr Blood Cancer. 2015;62:854–858. doi: 10.1002/pbc.25362. [DOI] [PubMed] [Google Scholar]
- 33.Prasher VP. Screening of medical problems in adults with Down syndrome Down Syndrome Research and Practice. 1994;2:59–66. [Google Scholar]
- 34.Zhu JL, Hasle H, Correa A, et al. Hospitalizations among people with Down syndrome: a nationwide population-based study in Denmark. Am J Med Genet A. 2013;161A:650–657. doi: 10.1002/ajmg.a.35711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Percy ME, Dalton AJ, Markovic VD, et al. Autoimmune thyroiditis associated with mild “subclinical” hypothyroidism in adults with Down syndrome: a comparison of patients with and without manifestations of Alzheimer disease. Am J Med Genet. 1990;36:148–154. doi: 10.1002/ajmg.1320360205. [DOI] [PubMed] [Google Scholar]
- 36.Evenhuis HM, van Zanten GA, Brocaar MP, Roerdinkholder WH. Hearing loss in middle-age persons with Down syndrome. Am J Ment Retard. 1992;97:47–56. [PubMed] [Google Scholar]
- 37.Berk AT, Saatci AO, Ercal MD, Tunc M, Ergin M. Ocular findings in 55 patients with Down’s syndrome. Ophthalmic Genet. 1996;17:15–19. doi: 10.3109/13816819609057864. [DOI] [PubMed] [Google Scholar]
- 38.Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol. 2014;32:1218–1227. doi: 10.1200/JCO.2013.51.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ness KK, Krull KR, Jones KE, et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St Jude Lifetime cohort study. J Clin Oncol. 2013;31:4496–4503. doi: 10.1200/JCO.2013.52.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ness KK, Armstrong GT, Kundu M, Wilson CL, Tchkonia T, Kirkland JL. Frailty in childhood cancer survivors. Cancer. 2015;121:1540–1547. doi: 10.1002/cncr.29211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bull MJ Committee on G. Health supervision for children with Down syndrome. Pediatrics. 2011;128:393–406. doi: 10.1542/peds.2011-1605. [DOI] [PubMed] [Google Scholar]
- 42.Hijiya N, Hudson MM, Lensing S, et al. Cumulative incidence of secondary neoplasms as a first event after childhood acute lymphoblastic leukemia. JAMA. 2007;297:1207–1215. doi: 10.1001/jama.297.11.1207. [DOI] [PubMed] [Google Scholar]
- 43.Nizetic D, Groet J. Tumorigenesis in Down’s syndrome: big lessons from a small chromosome. Nat Rev Cancer. 2012;12:721–732. doi: 10.1038/nrc3355. [DOI] [PubMed] [Google Scholar]
- 44.Hasle H, Friedman JM, Olsen JH, Rasmussen SA. Low risk of solid tumors in persons with Down syndrome. Genet Med. 2016;18:1151–1157. doi: 10.1038/gim.2016.23. [DOI] [PubMed] [Google Scholar]
- 45.Chicoine B, Roth M, Chicoine L, Sulo S. Breast Cancer Screening for Women With Down Syndrome: Lessons Learned. Intellectual and Developmental Disabilities. 2015;53:91–99. doi: 10.1352/1934-9556-53.2.91. [DOI] [PubMed] [Google Scholar]
- 46.Molgaard-Hansen L, Glosli H, Jahnukainen K, et al. Quality of health in survivors of childhood acute myeloid leukemia treated with chemotherapy only: a NOPHO-AML study. Pediatr Blood Cancer. 2011;57:1222–1229. doi: 10.1002/pbc.22931. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure: Cumulative incidence of chronic conditions by type of leukemia in patients with DS