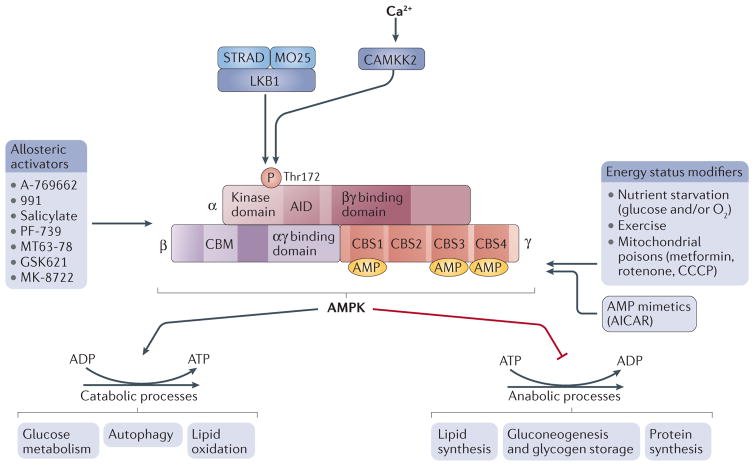
Figure 1. AMPK structure and activation.
Domain structure of the AMP-activated protein kinase (AMPK) trimer, showing the α-, β- and γ-subunits with their respective domains. The upstream kinases CAMKK2 and liver kinase B1 (LKB1) are shown above the AMPK complex. LKB1 is a heterotrimer composed of LKB1, STRAD and MO25; CAMKK2 is activated by intracellular calcium. Several factors lead to AMPK activation, such as mitochondrial poisons and oxygen or glucose starvation, as well as exercise. Drugs that activate AMPK include the AMP mimetic AICAR and several small-molecule allosteric activators (listed on the left-hand side). The effect of AMPK activation is to rewire metabolism to decrease anabolic processes (that is, ATP consumption) and increase catabolism (that is, ATP production) to restore a more favourable energy balance. Downstream substrates are grouped by biological functions. ATP-producing processes are activated, while ATP-consuming processes are inhibited. AID, auto-inhibitory domain; CBM, carbohydrate-binding module; CBS, cystathionine-β-synthase.