Figure 5. Modulation of the transcription of autophagy and lysosome genes by AMPK.
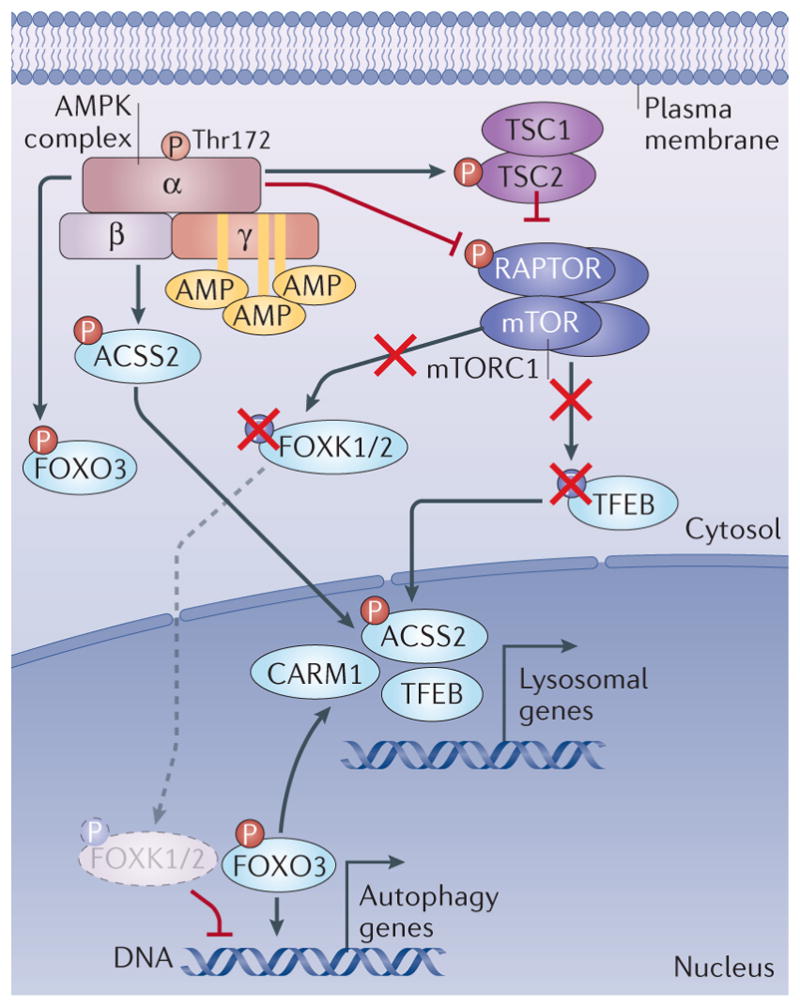
AMP-activated protein kinase (AMPK) regulates the activity of forkhead box protein O3 (FOXO3) through direct phosphorylation. In parallel, AMPK decreases mTOR complex 1 (mTORC1) (purple complex, composed of mTOR, RAPTOR, mLST8, DEPTOR and PRAS40; not all shown) activity through phosphorylation of RAPTOR and the upstream regulator TSC2. This leads to downregulation of mTORC1 activity. This results in dephosphorylation of mTORC1 targets FOXK1 and FOXK2, as well as transcription factor EB (TFEB). As a result, dephosphorylated FOXK1 and FOXK2 can no longer act as transcriptional repressors of FOXO3 targets, allowing higher transcription of autophagy genes downstream of FOXO3 binding. However, dephosphorylation of TFEB allows its nuclear translocation and activation of its target genes, including genes involved in lysosome biogenesis. In addition, an increased CARM1 protein level resulting from FOXO3-dependent gene activation further enhances TFEB-dependent gene expression. Acetyl-CoA carboxylase 2 (ACC2) phosphorylation by AMPK stimulates its nuclear import where it enhances TFEB target gene expression.
