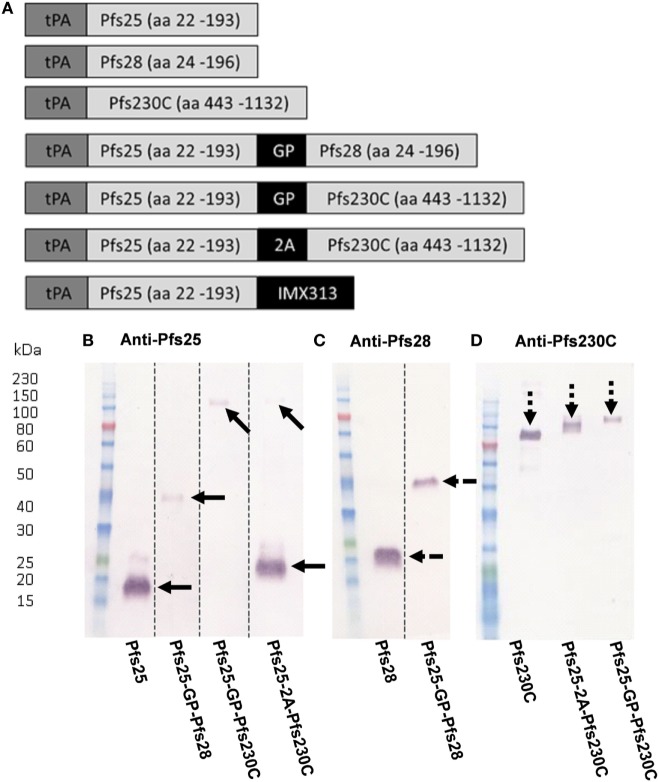
Figure 1.
Viral vector construct generation and validation. (A) Seven constructs were used to generate recombinant ChAd63 and modified Vaccinia Ankara (MVA) viral vectors expressing single or multiple antigens. All antigens are fused to an N-terminal secretion signal peptide tissue plasminogen activator (tPA). Dual-antigen constructs link antigens with glycine–proline (GP) linker or a 2A linker. Last construct fused Pfs25 to the oligomerization domain IMX313. (B–D) Expression of antigens in suspension HEK293 cells. The pENTR4-LPTOS entry vector expressing the following in each lane represented: Pfs25 (18.81 kDa); Pfs25-GP-Pfs28 (38.27 kDa); Pfs25-GP-Pfs230C (98.89 kDa); Pfs25-2A-Pfs230C (19.26 kDa (Pfs25) and 83.5 kDa (Pfs230C)); Pfs28 (19.04 kDa); and Pfs230C (83.5 kDa), respectively, were used to transfect HEK293 cells, and the supernatant was harvested 4 days posttransfection. 15 µl of supernatant was loaded per lane, and after staining, the western blots were developed for 5 min. The figure shows western blots using day 70 anti-serum (pooled from five mice) collected after ChAd63-MVA vaccination against the respective antigens (B) Pfs25, (C) Pfs28, and (D) Pfs230C. Dotted lines indicate cut lanes from original blot (B,C) with the corresponding original blots shown in Figure S2 in Supplementary Material. The arrows represent the positions of the observed bands against Pfs25 (solid arrows), Pfs28 (broken arrows), and Pfs230C (dashed arrow).