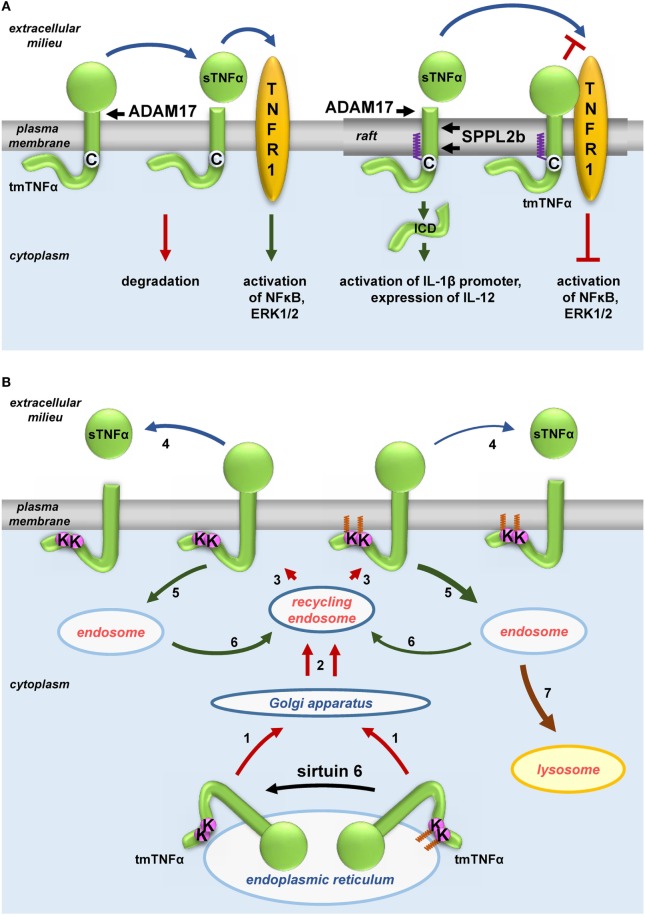
Figure 3.
Influence of fatty acylation of transmembrane tumor necrosis factor α (TNFα) on production of soluble sTNFα. (A) S-palmitoylation and (B) ε-N-myristoylation of tmTNFα. (A) Non-palmitoylated tmTNFα is localized outside rafts while that S-palmitoylated on Cys30—in rafts of the plasma membrane. tmTNFα is cleaved by ADAM17 protease in both these plasma membrane environments giving rise to sTNFα, which subsequently activates TNF receptor (TNFR) 1 receptor leading to activation of NFκB and ERK1/2. However, only the raft-residing tmTNFα is further processed by SPPL2b protease to yield ICD, which activates the promoter of interleukin (IL)-1β and expression of IL-12. On the other hand, a pool of S-palmitoylated tmTNFα interacts in rafts with TNFR1 preventing its activation by sTNFα. (B) tmTNFα is transported from the endoplasmic reticulum via Golgi apparatus and recycling endosomes [1, 2] to the plasma membrane [3]. In the plasma membrane, TNFα is cleaved by ADAM17 giving rise to sTNFα [4] or is internalized [5] and either returns from the endosomes to the plasma membrane [6, 3] or is directed to lysosomes for degradation [7]. ε-N-myristoylation of tmTNFα at Lys19 and Lys20 facilitates its degradation [5, 7] at the expense of processing to sTNFα [4]. Oligomerization of tmTNFα and TNFR1 is not shown.