Figure 1.
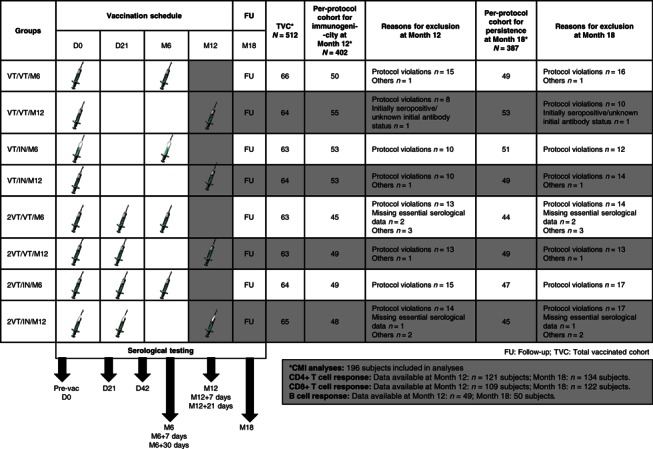
Study design diagram + CONSORT. Explanation for the study design: In the primary study, subjects were randomized into 8 study groups to receive one or two doses (21 days apart) of AS03A‐adjuvanted H5N1 vaccine with either A/Vietnam/1194/2004 strain (VT) or A/Indonesia/05/2005 strain (IN). A homologous or heterologous booster dose (VT or IN) was administered to 4 study groups at Month 6 and the remaining 4 study groups at Month 12. Blood samples were drawn before vaccination, at Days 21 and 42, Month 6 (+7 and +30 days), Month 12 (+7 and +21 days), and at Month 18. This manuscript presents data from groups that were boosted at Month 12. Group names: VT/VT/M12: A/Vietnam/1194/2004 vaccine at Day 0; A/Vietnam/1194/2004 vaccine at Month 12. VT/IN/M12: A/Vietnam/1194/2004 vaccine at Day 0; A/Indonesia/05/2005 vaccine at Month 12. 2VT/VT/M12: A/Vietnam/1194/2004 vaccine at Days 0 and 21; A/Vietnam/1194/2004 vaccine at Month 12. 2VT/IN/M12: A/Vietnam/1194/2004 vaccine at Days 0 and 21; A/Indonesia/05/2005 vaccine at Month 12.
