Abstract
Objective
To evaluate the safety of CSL's split‐virion inactivated trivalent 2009 Southern Hemisphere formulation influenza vaccine (TIV) in children.
Methods
We enrolled 1992 healthy children into three groups: Cohorts A, ≥6 months to <3 years; B, ≥3 years to <9 years; and C, ≥9 years to <18 years. Children received one or two doses of 0·25 ml (22·5 μg haemagglutinin) or 0·5 ml (45 μg) TIV, depending on age and prior vaccination history. We collected post‐vaccination solicited adverse event (AE) data (days 0–6), including fever (temperature: ≥37·5°C axilla, ≥38·0°C oral), unsolicited AEs (days 0–29) and serious AEs (SAEs) and new‐onset chronic illnesses (NOCIs; to day 180 after last vaccination).
Results
At least one solicited AE was reported by 80%/78%/78% of children in Cohorts A, B and C, respectively. Systemic AEs were more common among Cohort A (72% of participants), and local AEs were more common among Cohort C (71% of participants). Fever was more common in younger cohorts, in influenza vaccine‐naïve children (29% of Cohort A receiving their first dose), and following first compared with second doses. Severe fever following a first dose prevented 20 participants receiving their second scheduled vaccine dose. A 7‐month‐old participant had a single uncomplicated febrile convulsion on the day of vaccination.
Conclusions
Nearly 80% of subjects reported at least one solicited AE following immunization. Fever prevalence was highest in vaccine‐naïve Cohort A participants, similar to other paediatric studies using CSL vaccine. Further research to understand fever‐related AEs in children following CSL's TIV is recommended.
Keywords: Adverse events, fever, influenza vaccine, paediatrics, safety
Introduction
Influenza is a serious public health problem predictably causing annual epidemics of infection, hospitalization, economic burden and deaths. Children have the highest rates of infection, have the highest rates of hospitalization due to laboratory‐confirmed influenza and are the principal agents of transmission in communities.1, 2, 3, 4 Annual vaccination is the most effective method for preventing influenza and its complications. Influenza vaccines have been used in adult populations for many decades, but the focus on their use in children, for direct5, 6 and indirect protection,4, 7, 8, 9, 10, 11, 12 is a more recent phenomenon. This focus has led to a number of recommendations for universal annual vaccination in healthy children.13, 14 Following a cluster of three paediatric deaths in Western Australia (WA) during 2007, a State Government influenza vaccination programme was established for children aged ≥6 months to <5 years from 2008, with free vaccine provided by the WA Government in conjunction with CSL Biotherapies and Sanofi Pasteur.15 Seasonal influenza vaccine has been recommended for Australians ≥6 months of age with medical conditions predisposing to severe influenza for many years, and this became publicly funded nationally for this cohort in 2010.16
There is a relative lack of published safety information on the use of influenza vaccines in children, particularly for younger cohorts.17 At the time of this study, there was one published study reporting safety data for CSL's seasonal trivalent split‐virion inactivated influenza vaccine (Fluvax®; CSL Ltd, Parkville, Vic., Australia) in children.18 A US controlled trial was also commenced during the conduct of this study, with results now available at ClinicalTrials.gov (Table 1).19 With this study, we sought to expand the available safety data for the use of seasonal influenza vaccine in children.
Table 1.
Percentage (with 95% confidence intervals) of participants reporting fevera of any severity in the 7 days (day 0 to day 6) following administration of CSL's seasonal trivalent influenza vaccine, and one control comparator, by dose and age group
| Study and vaccine | Dose | Age cohorts | ||
|---|---|---|---|---|
| ≥6 months to <3 years | ≥3 to <9 years [≥3 to <5 years/≥5 to <9 years] | ≥9 to <18 years | ||
| Study: Nolan et al.18 , b | ||||
| 2005 trivalent Southern Hemisphere vaccine | 2005 | n = 151 | n = 147 | n = 0c |
| Dose one | 23 (17–31) |
16 (11–23) [23 (15–35)/10 (5–18)] |
– | |
| Dose two | 23 (17–30) |
8 (5–14) [(17 (10–29)/1 (0–7)] |
– | |
| 2006 trivalent Southern Hemisphere vaccine | 2006 | n = 76 | n = 197 | n = 0c |
| Single booster dose | 39 (29–51) |
27 (21–33) [41 (32–50)/12 (7–21)] |
– | |
| Study: safety profile of CSL Limited's influenza virus vaccine compared to a US licensed comparator influenza virus vaccine19 | ||||
| 2009/2010 trivalent Northern Hemisphere vaccine | n = 231 | n = 254 | n = 254 | |
| Dose one | 37 (31–43) |
22 (17–28) [32 (23–43)/16 (11–22)] |
6 (4–10) | |
| Dose two | 15 (11–20) |
2 (1–5) [14 (9–23)/0 (0–2)] |
–d | |
| Comparator split‐virion vaccine (Fluzone) | n = 228 | n = 257 | n = 250 | |
| Dose one | 14 (10–19) |
9 (6–13) [11 (6–19)/9 (6–14)] |
4 (2–7) | |
| Dose two | 14 (10–19) |
2 (1–4) [16 (10–25)/2 (1–5)] |
–d | |
| This study | ||||
| 2009 trivalent Southern Hemisphere vaccine | n = 710 | n = 880 | n = 402 | |
| Dose one | 29 (26–32) |
19 (17–22) [28 (24–33)/14 (11–17)] |
5 (3–8) | |
| Dose two | 18 (15–21) |
10 (8–12) [13 (10–17)/7 (5–10)] |
–d | |
Methods
Study design
This prospective, multicentre, open‐label, uncontrolled phase IV clinical trial (ClinicalTrials.gov: NCT00825162) was conducted at seven Australian sites during the 2009 Southern Hemisphere autumn. The primary objective of the study was to evaluate the safety and tolerability of the trivalent inactivated influenza vaccine (Fluvax® and Fluvax Junior®; CSL Limited) in infants, children and adolescents. The study was conducted in accordance with the International Conference on Harmonization Guideline for Good Clinical Practice. The study protocol was approved by the Human Research Ethics Committee at each study centre, and written informed consent was obtained from each participant's parent/guardian prior to any study procedures. Participant assent was also obtained from those capable of making an informed decision about their own participation, in accordance with local institutional ethics committee guidelines.
Study participants
Healthy children aged ≥6 months to <18 years were eligible to participate. Exclusion criteria included an allergy to egg products or any vaccine component; evidence of an active infection; receipt of an experimental or seasonal influenza vaccine in the previous 6 months; a confirmed or suspected immunosuppressive condition; a history of Guillain–Barré syndrome; a major congenital defect or serious illness; a history of neurologic disorders or seizures (single seizure events more than 2 years previously permitted); receipt of immunoglobulins or blood products within the previous 3 months; participation in a clinical study or use of an investigational compound within the previous 3 months; receipt of recent immunosuppressive and immunomodulatory medication, including systemic corticosteroids; and treatment with cytotoxic drugs. Children aged ≥6 months to <9 years had to be born at or after 36 weeks of gestation to be eligible for enrolment, and adolescent females ≥9 years of age were required to provide a negative pregnancy test prior to vaccination.
Participants were stratified into three cohorts based on age at time of enrolment with number of doses and volume (0·25/0·5 ml) determined by cohort and prior immunization history (Table S1).
Study vaccine
The 2009 Southern Hemisphere trivalent inactivated influenza vaccine was used, manufactured at CSL's plant in accordance with the registered procedure. Each 0·5 ml of vaccine contained 15 μg of each of the following haemagglutinin antigens: A/Brisbane/59/2007 (H1N1), A/Brisbane/10/2007 (H3N2) and B/Florida/4/2006, as recommended by the World Health Organization for the 2009 Southern Hemisphere influenza season. The study vaccine virus was propagated in the allantoic fluid of embryonated chicken eggs. Following harvest of virus, the following steps were used to produce a purified split‐virion vaccine: purification using a sucrose gradient, inactivation with β‐propiolactone, virus splitting using sodium taurodeoxycholate, purification and suspension in a phosphate‐buffered isotonic solution. The vaccine is thiomersal free.
Study procedures
Study visits were conducted on days 0 (dose one), 30 + 4 (exit visit for single‐dose participants or administration of dose two), 60 + 4 (two‐dose participants exit visit only), with a final safety telephone contact at 180 + 14 days following the last vaccine dose. A medical examination was performed during visits, and a 30‐minutes observation period followed vaccination.
Parents used diary cards to record solicited local and systemic AEs on the day of vaccination and during the 6 days following (Table S2); unsolicited AEs and any medications taken on the day of vaccination and during the 29 days following; and medication used to treat and medical visits for an AE. The occurrence of serious AEs (SAEs) and new‐onset chronic illnesses (NOCIs) was assessed up to 180 days following the last vaccine dose. An SAE was any untoward medical occurrence resulting in death, which was life‐threatening, required an unexpected inpatient hospitalization (at least 24 hours) or prolongation (by at least 24 hours) of existing hospitalization; resulted in persistent or significant disability/incapacity; was a congenital anomaly/birth defect; and/or was judged by the treating physician to be medically significant. Adverse event severity was graded as mild (Grade 1), moderate (Grade 2) or severe (Grade 3), following parent training (Table S2).
All solicited local AEs were considered related to the study vaccine. Clinical significance and causality of solicited systemic AEs, all unsolicited AEs and NOCIs were assigned by the site investigator.
A severe fever (Table S2) ≥40·0°C (oral) or ≥39·5°C (axillary) within 48 hours of vaccination was one of the contraindications to administering further doses of study vaccine. Oral and axillary temperatures are presented as combined data.
Statistical analyses and power
The number and proportion (with 95% confidence intervals) of participants with an AE following dose one and dose two are summarized using a safety population comprising all participants who received the relevant dose and provided at least one safety assessment after vaccination.
We planned for a safety population of 2025 participants: Cohort A, 810 participants; Cohort B, 810; and Cohort C, 405. Based on expected enrolment, AEs occurring at a rate of 1 in 1000 participants had an 86·8% probability of being detected, and AEs with rates of 1 in 676 had a 95% chance of being observed.
Results
Participants
Informed consent was provided for 2024 potential participants, and of these, 1992 were enrolled and stratified by age: Cohort A, 710 members; Cohort B, 880 members; and Cohort C, 402 members (Tables 2 and S1, Figure 1). Despite considerable efforts during the available enrolment window prior to the commencement of the local influenza season, fewer Cohort A participants were enrolled than planned; but numbers were felt sufficient to still enable meaningful analysis of the safety data. Within the enrolled cohorts (A/B/C), AEs occurring at a rate of 1 in 300 had a 90·7/94·7/73·9% chance of detection and AEs with a rate of 1 in 238/294/135 had a 95% chance of being observed, respectively.
Table 2.
Participant characteristics
| Characteristics | Cohort A (n = 710) | Cohort B (n = 880) | Cohort C (n = 402) |
|---|---|---|---|
| Mean age, years (standard deviation) | 1·84 (0·74) | 5·54 (1·66) | 12·64 (2·48) |
| Female sex, n (%) | 45·8 (325) | 49·9 (439) | 50·2 (202) |
| Previous receipt of any influenza vaccination, n (%) | 10·0 (71) | 29·5 (260) | 22·6 (91) |
| Receipt of influenza vaccine prior to 2008, n (%) | 3·2 (23) | 20·1 (177) | 16·2 (65) |
| Receipt of influenza vaccine during 2008, n (%) | 8·6 (61) | 17·4 (153) | 13·4 (54) |
Figure 1.
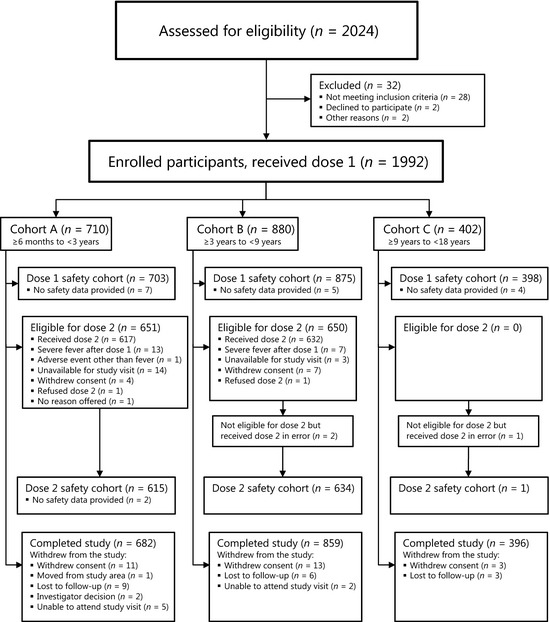
Study design and participant designation.
The first subject was vaccinated on 6 March 2009, the final dose one was given on 16 July 2009, the final dose two on 24 August 2009, and the final day 180 telephone call was made on 22 February 2010. The first Australian case of influenza A(H1N1)pdm09 virus was confirmed in Brisbane on 9 May 2009,20 and virus circulation became rapidly widespread in all site cities.21
A total of 55 participants withdrew (Figure 1) from the study (Cohort A: 28; Cohort B: 21; and Cohort C: 6). A further 17 subjects (Cohort A: 10 and Cohort B: 7) were initially classified as withdrawn, but went on to provide final safety data at the day 180 telephone contact – therefore, not meeting the study definition of a withdrawn subject. These 17 subjects had AEs following dose one: fever (axillary temperature ≥39·5°C within 48 hours of vaccination, n = 15), influenza‐like illness (n = 1) and urticarial rash (n = 1). The 15 participants with a severe fever AE following dose one were part of a larger group of 20 participants (Figure 1) who did not receive a scheduled dose two due to a severe fever AE following dose 1 (Cohort A: 13 and Cohort B: 7). These 20 subjects did not meet the definition of a withdrawn subject as they provided ongoing safety data until study conclusion.
Thirteen percent of participants had received influenza vaccine during the 2008 season, and 13% had received vaccine at any time prior to 2008.
Safety and tolerability
Solicited local adverse events
Solicited local AEs were common (Table 3) and were mostly of mild/moderate severity (Table 4) and short‐lived. Solicited local AEs were more common in older children and decreased within cohorts following dose two: reported by 36·1%, 58·5% and 70·6% of participants in Cohorts A, B and C, respectively, after the first vaccination, and 27·2% and 49·2% of participants in Cohorts A and B, respectively, following dose two (Table 4). Injection site pain was the most commonly reported local AE in all cohorts.
Table 3.
Summary of adverse events (AE) and fever‐related events in the safety populations after first/second dose of vaccine
| AEs in study safety cohorts | Cohort A (n = 703) % (n) | Cohort B (n = 875) % (n) | Cohort C (n = 398) % (n) | Total (n = 1976) % (n) |
|---|---|---|---|---|
| One or more AEs | 91·5 (643) | 87·7 (767) | 84·7 (337) | 88·4 (1747) |
| Eligible but did not receive dose two due to AE | 1·8 (13) | 0·8 (7) | 0 | 1·0 (20) |
| Solicited AEsa | 80·1 (563) | 78·2 (684) | 77·9 (310) | 78·8 (1557) |
| Any solicited local AEs | 43·4 (305) | 66·9 (585) | 70·6 (281) | 59·3 (1171) |
| Any solicited systemic AEs | 71·7 (504) | 46·6 (408) | 42·7 (170) | 54·8 (1082) |
| Unsolicited AEsb | 75·5 (531) | 59·5 (521) | 42·0 (167) | 61·7 (1219) |
| Deaths | 0 | 0 | 0 | 0 |
| Serious AEs (SAEs) | 2·7 (19) | 0·6 (5) | 0·5 (2) | 1·3 (26) |
| SAEs considered related to vaccinec | 0 | 0 | 0 | 0 |
| Withdrawal due to an SAE | 0 | 0 | 0 | 0 |
| New‐onset chronic illnesses (NOCIs)d | 1·4 (10) | 0·6 (5) | 0·5 (2) | 0·9 (17) |
| NOCIs considered related to vaccinec | 0·3 (2) | 0 | 0 | 0·1 (2) |
| Fever‐related events | ||||
| Subject unable to receive prescribed dose 2 due to severe fever AE following dose 1 | 1·8 (13) | 0·8 (7) | –e | 1·5 (20)f |
| Febrile convulsion following immunization | 0·1 (1) | 0 | 0 | 0·1 (1) |
Solicited AEs from day 0 to day 6 following vaccine.
Unsolicited AEs include: unsolicited AEs from day 0 to day 29 following vaccine and SAEs/NOCIs from day 0 to day 180 following the last dose of vaccine.
Related AEs are those assessed by an investigator as having a causality of unknown, possibly, probably or definitely related to study vaccine.
Three Cohort A children each had two NOCI events, meaning a total of 20 NOCIs were reported in 17 children.
Second dose not given to Cohort C.
Denominator: Cohort A and Cohort B subjects eligible for dose 2 (n = 1301).
Table 4.
Any and Grade 3 solicited local and systemic adverse events (AE) within 6 days after administration of study vaccine
| AE | Cohort A % (n) | Cohort B % (n) | Cohort C % (n) | ||
|---|---|---|---|---|---|
| Dose 1 | Dose 2 | Dose 1 | Dose 2 | Dose 1 | |
| Any local solicited AE | 36·1 (254) | 27·2 (167) | 58·5 (512) | 49·2 (312) | 70·6 (281) |
| Any Grade 3 event | 0·6 (4) | 0·5 (3) | 3·5 (31) | 2·1 (13) | 3·8 (15) |
| Pain | 22·8 (160) | 18·0 (111) | 52·9 (463) | 44·5 (282) | 68·1 (271) |
| Grade 3 | 0·1 (1) | 0·3 (2) | 0·2 (2) | 0·3 (2) | 0·3 (1) |
| Redness (erythema) | 22·1 (148) | 17·7 (109) | 21·6 (189) | 17·2 (109) | 16·6 (66) |
| Grade 3 | 0·3 (2) | 0·2 (1) | 2·1 (18) | 1·1 (7) | 2·0 (8) |
| Injection site swelling (induration) or lump | 9·4 (66) | 10·4 (64) | 15·7 (137) | 12·1 (77) | 13·1 (52) |
| Grade 3 | 0·3 (2) | 0 | 2·2 (19) | 1·3 (8) | 3·0 (12) |
| Any systemic solicited AE | 60·3 (424) | 42·4 (261) | 39·5 (346) | 24·8 (157) | 42·7 (170) |
| Any Grade 3 event | 5·7 (40) | 2·3 (14) | 2·7 (24) | 2·1 (13) | 1·0 (4) |
| Fever | 28·6 (201) | 17·9 (110) | 19·5 (171) | 9·9 (63) | 5·0 (20) |
| Grade 3 | 1·8 (13) | 1·0 (6) | 0·8 (7) | 0·3 (2) | 0 |
| Headache | 3·6 (25) | 2·0 (12) | 15·5 (136) | 6·0 (38) | 26·9 (107) |
| Grade 3 | 0·1 (1) | 0 | 0·5 (4) | 0·6 (4) | 0·5 (2) |
| Myalgia | 3·7 (26) | 2·6 (16) | 9·9 (87) | 5·4 (34) | 20·1 (80) |
| Grade 3 | 3 (0·4) | 0·2 (1) | 3 (0·3) | 0·5 (3) | 0 |
| Nausea/vomiting | 11·2 (79) | 5·0 (31) | 8·6 (75) | 3·9 (25) | 5·3 (21) |
| Grade 3 | 1·7 (12) | 0·7 (4) | 0·7 (6) | 0·8 (5) | 0·3 (1) |
| Diarrhoea | 14·2 (100) | 8·5 (52) | 4·6 (40) | 3·2 (20) | 5·3 (21) |
| Grade 3 | 0·6 (4) | 0·5 (3) | 0·2 (2) | 0 | 0·3 (1) |
| Loss of appetite (Cohort A only) | 20·1 (141) | 13·3 (8·2) | – | – | – |
| Grade 3 | 1·1 (8) | 0·7 (4) | – | – | – |
| Irritability (Cohort A only) | 42·0 (295) | 28·5 (175) | – | – | – |
| Grade 3 | 3·4 (24) | 1·1 (7) | – | – | – |
| Malaise (Cohorts B and C only) | – | – | 20·5 (179) | 11·4 (72) | 16·6 (66) |
| Grade 3 | – | – | 1·6 (14) | 1·6 (10) | 0·3 (1) |
Solicited systemic adverse events
The frequency of solicited systemic AEs (Table 4) following dose one was highest in Cohort A (60·3%), was similar in Cohorts C (42·7%) and B (39·5%) and fell with a second dose in both Cohorts A (42·4%) and B (24·8%).
Fever was more common in the younger cohorts, in those who had not previously received any influenza vaccine and in those receiving a first study dose compared with those receiving the second study dose (Table 4).
To allow comparison with data from other studies, Cohort B was divided into children aged ≥3 to <5 and children aged ≥5 and <9 years (Table 1). Fever of severe intensity following dose 1 was reported by 13 participants in Cohort A (1·8%) and seven participants in Cohort B (0·8%), including a 7‐month‐old participant (Cohort A) who had a febrile convulsion within 4 hours of receiving dose one. This participant experienced fever of 39·9°C (axilla) and severe vomiting on the day of and the day following dose one. She was seen in a hospital emergency department, but not admitted, and treated with paracetamol, ibuprofen and oral rehydration solution. The fever and vomiting had concluded on the day following vaccination.
There were 588 episodes of fever of any intensity following vaccination: 389 (66%) of Grade 1 fever, 171 (29%) of Grade 2 and 28 (4%) of Grade 3 (Table 5). When fever did occur, it was managed using antipyretics on 57% of occasions, and resulted in a healthcare visit on 14% of occasions. Details on the duration of fever by grade, antipyretic treatment and healthcare use in response to fever are provided (Table 5).
Table 5.
Fever severity, mean and median duration, use of antipyretics/medical services, by dose number and history of previous influenza vaccination
| Fever, all participants | Cohort A, % (n) | Cohort B, % (n)a | Cohort C, % (n)b | ||
|---|---|---|---|---|---|
| Dose 1 | Dose 2 | Dose 1 | Dose 2 | Dose 1 | |
| Grade 1 | 17·5 (123) | 13·2 (81) | 14·3 (125) | 6·9 (44) | 4·0 (16) |
| Grade 2 | 9·2 (65) | 3·7 (23) | 4·5 (39) | 2·7 (17) | 1·0 (4) |
| Grade 3 | 1·8 (13) | 1·0 (6) | 0·8 (7) | 0·3 (2) | 0 |
| Any | 28·6 (201) | 17·9 (110) | 19·5 (171) | 9·9 (63) | 5·0 (20) |
| Mean/median duration (days) of fever | |||||
| Duration of total fever of any grade | 1·54/1·00 | 1·67/1·00 | 1·40/1·00 | 1·58/1·00 | 1·21/1·00 |
| Use of antipyretics to manage fever by Grade | |||||
| Grade 1 | 8·4 (59) | 4·7 (29) | 6·9 (60) | 2·5 (16) | 0·5 (2) |
| Grade 2 | 8·1 (57) | 3·7 (23) | 3·7 (32) | 2·2 (14) | 0·7 (3) |
| Grade 3 | 1·8 (13) | 0·8 (5) | 0·8 (7) | 0·3 (2) | 0 |
| Any | 18·3 (129) | 9·3 (57) | 11·3 (99) | 5·0 (32) | 1·3 (5) |
| Medically attended fever by Grade | |||||
| Grade 1 | 0·8 (6) | 1·5 (9) | 0·7 (6) | 0·8 (5) | 0 |
| Grade 2 | 2·6 (18) | 1·3 (8) | 0·8 (7) | 1·1 (7) | 0 |
| Grade 3 | 0·8 (6) | 0·5 (3) | 0·1 (1) | 0·3 (2) | 0 |
| Any | 4·3 (30) | 3·3 (20) | 1·6 (14) | 2·2 (14) | 0 |
| Fever, no previous influenza vaccination | |||||
| Grade 1 | 18·0 (114) | 13·1 (79) | 16·2 (100) | 7·2 (43) | 4·5 (14) |
| Grade 2 | 10·1 (64) | 3·8 (23) | 5·5 (34) | 2·8 (17) | 1·0 (3) |
| Grade 3 | 2·1 (13) | 1·0 (6) | 1·0 (6) | 0·3 (2) | 0 |
| Any | 30·2 (191) | 17·9 (108) | 22·7 (140) | 10·3 (62) | 5·5 (17) |
| Fever, previous influenza vaccination | |||||
| Grade 1 | 12·7 (9) | 16·7 (2) | 9·8 (25) | 3·0 (1) | 2·3 (2) |
| Grade 2 | 1·4 (1) | 0 | 2·0 (5) | 0 | 1·1 (1) |
| Grade 3 | 0 | 0 | 0·4 (1) | 0 | 0 |
| Any | 14·1 (10) | 16·7 (2) | 12·1 (31) | 3·0 (1) | 3·4 (3) |
Excludes one Cohort B member whose prior vaccination status was not available.
Excludes the dose two data for one Cohort C member who was administered a second dose in error.
Of the 392 fever episodes of any intensity reported on the day of or the 6 days following dose 1, fever commenced on the same day as vaccination in 178 (45%) and on the next day in 143 (36%). For the 173 fever episodes following dose 2, the equivalent values are 55 (32%) and 29 (17%), respectively.
Unsolicited adverse events
There were no deaths in study participants (Table 3). Of 26 SAEs, none were assessed as causally related to vaccine by investigators. Seventeen subjects reported 20 NOCIs; two were considered possibly vaccine‐related by investigators: food allergy and eczema.
Unsolicited AEs were common, reported by 61·7% of total participants: Cohort A: 75·5% of participants; Cohort B: 59·5%; and Cohort C: 42·0% (Table 3).
In each cohort, the most common unsolicited AE was upper respiratory tract infection (Cohort A: 29·6%; Cohort B: 16·2%; and Cohort C: 8·3%). As there was no viral testing conducted during respiratory tract illness in this study, it is not possible to report association of these illnesses with influenza A(H1N1)pdm09 or other respiratory viruses.
Discussion
In this study of CSL's 2009 Southern Hemisphere TIV, we found AEs to be common, with 59% of children having at least one solicited local AE and 55% of children having at least one solicited systemic AE following immunization. Most AEs were graded as mild or moderate in severity, with Grade 3 AEs uncommon. Twenty participants in Cohorts A and B did not receive a scheduled second dose of study vaccine as per protocol due to Grade 3 fever following dose one, with one‐7‐month‐old experiencing a single febrile convulsion on the day of vaccine administration. When present, fever typically began shortly after immunization: in 82% of those who reported fever following dose 1, it commenced on the same or day following vaccination; and in 49% for dose 2. Two NOCIs were considered possibly vaccine‐related (food allergy and eczema): neither is listed as a possible AE in the product information, and there is no clear causal association between TIV and these AEs.
We report our findings in the context of an increased focus on the safety, particularly fever‐related AEs, of CSL's trivalent seasonal influenza vaccine in children.22 CSL's 2010 Southern Hemisphere trivalent influenza vaccine was associated with the rapid onset of fever in children, with febrile convulsions in some. Results from this study were provided to the Australian Government by CSL Limited to assist with the initial safety review of the 2010 events. From this study, severe and any graded, fever was highest in vaccine‐naïve children in the youngest cohort, providing some confidence that the severe fever AEs seen in 2010 were unlikely to be due to repeated annual doses of CSL's seasonal TIV vaccine.
In subsequent findings from the WA programme, those aged 6–59 months who received CSL's 2010 TIV had a fever prevalence of 56·5%, compared with 17·3% in children receiving another brand of TIV, as reported retrospectively by their parents most likely blinded to vaccine brand.22 Further, in the 49 days following WA programme commencement in 2010, 38 febrile convulsions were identified as occurring within 72 hours of vaccine administration.22 Despite the prevalence of fever AEs associated with CSL's 2009 TIV in this study, the rate of febrile convulsions following administration of CSL TIV in young children reported from WA was significantly lower in 2009 and 2008, when compared to 2010.22 The prevalence estimates for febrile convulsions following administration of the CSL Southern Hemisphere 2010 preparation range from a conservatively estimated value for all children <5 years of age 8·1 per 1000 doses (Fluvax Junior),22 to approximately one in 100.23
The proportion of participants reporting any fever in this study fell within the range reported in the two studies (one reported on ClinicalTrials.gov) with safety data available for CSL's TIV in children (Table 1).18, 19 Fever following dose one/two for Cohort A (29%/18%) and Cohort B (20%/10%) was somewhat higher for dose one compared to the 2005 Southern Hemisphere formulation: Cohort A (23%/23%) and Cohort B (16%/8%), but lower than the values for the single dose of 2006 Southern Hemisphere formulation: Cohort A (39%) and Cohort B (27%).18 Dose one values were lower than the 2009/2010 Northern Hemisphere formulation in Cohort A (dose one/two: 37%/15%), but similar for Cohorts B (22%/2%) and C (6%/–) (Tables 1 and 4).19 From that same study, dose one values for the control split‐virion vaccine were consistently lower than any of the CSL preparations, including the one used in this study (Table 1).19 Fever was also more common with the 30 μg (double) dose of CSL's 2009 monovalent influenza A(H1N1) vaccine: in an uncontrolled Australian study, for all participants (≥6 months to <9 years) following dose one any fever occurred in 24% (15 μg haemagglutinin) and 41% (30 μg) of recipients, and following dose two, 18% and 14%, respectively.24 From a placebo‐controlled US study using the same product, the proportion of participants with any fever in Cohort A (≥6 months to <3 years) following dose one or two was 23% (placebo arm), 25% (7·5 μg) and 43% (15 μg), for Cohort B (≥3 to <9 years) 14% (placebo), 19% (7·5 μg) and 20% (15 μg).25
Whilst there is no definitive explanation for the increased reactogenicity of CSL's 2010 Southern Hemisphere TIV, or CSL's other seasonal TIVs, a number of potential causes have been put forward.26 One possible contributing factor, inadequate splitting of vaccine virions by sodium taurodeoxycholate, may be intermittent.27 Analyses conducted by Blyth et al.28 as well as by CSL29 indicate that the CSL TIVs, as a class, generally induce higher levels of cytokines in in vitro paediatric peripheral blood mononuclear cells (PBMCs) and whole blood cell assays, and that this resulted from a viral‐derived, heat‐labile component, and not bacterial contamination.29 Based on current information, it is thought that increased AEs seen in children receiving CSL's 2010 Southern Hemisphere TIV resulted from the combination of differences in the method of manufacture of the CSL TIVs, as compared to other TIV manufacturers, and the effect of the newly introduced H1N1 strain combined with the particular B strain recommended that season.29
As a result of the higher than expected rates of febrile convulsions in children receiving CSL's 2010 Southern Hemisphere TIV, CSL TIVs are not currently approved for use in children <5 years of age and not recommended for children aged 5 to <10 years in Australia if alternative vaccines are available.
In this study using CSL's 2009 Southern Hemisphere TIV, we found fever was more common than a 2009/2010 Northern Hemisphere split‐virion comparator in a US study, as described in data available on ClinicalTrials.gov.19 Further work continues on attempting to identify the issue or issues responsible for the increased reactogenicity of CSL's TIV in children.
Author's contributions
Michael Lai, Gunter Hartel, Wilson Hu and Stephen Lambert were responsible for study concept and design. Stephen Lambert, Raymond Chuk, Michael Nissen, Terry Nolan, Jodie McVernon, Robert Booy, Leon Heron, Peter Richmond, Tony Walls, Helen Marshall and Graham Reynolds were responsible for data acquisition. Gunter Hartel, Wilson Hu, Michael Lai and Stephen Lambert were responsible for data analysis, and all authors were responsible for data interpretation. Stephen Lambert drafted the manuscript. All authors contributed to critical revision of the manuscript and have seen and approved the final version of the manuscript. Michael Lai was medical monitor for the study.
Declarations of interests
This study was sponsored by CSL Limited. Assistant Professor Lambert reported not having shares, paid employment or consultancies with CSL Limited; being an investigator on vaccine and epidemiological studies sponsored by CSL Limited and other companies; receiving support for conference attendance from GlaxoSmithKline and CSL Limited; and being a member of vaccine advisory boards for GlaxoSmithKline, Wyeth/Pfizer and Sanofi Pasteur. Dr Chuk reported not having shares, paid employment or consultancies with CSL Limited; being an investigator on vaccine and epidemiological studies sponsored by CSL Limited and other companies; received support for conference attendance from GlaxoSmithKline. Assistant Professor Nissen reported not having shares, paid employment or consultancies with CSL Limited; being an investigator on vaccine and epidemiological studies sponsored by CSL Limited and other companies; receiving support for conference attendance from GlaxoSmithKline, Novartis and Wyeth/Pfizer; being a member of vaccine advisory boards for GlaxoSmithKline, Wyeth/Pfizer and Novartis; and being a member of the Australian Technical Advisory Group on Immunisation. Professor Nolan reported not having shares, or paid employment with CSL Limited; receiving travel assistance and honoraria for meetings of scientific experts to advise on the design and interpretation of studies of a CSL experimental influenza vaccine in the elderly in 2007/8; being an investigator on vaccine and epidemiological studies sponsored by CSL Limited and other companies; receiving support for conference attendance to present scientific data from GlaxoSmithKline and CSL Limited; being a member of vaccine research advisory boards for GlaxoSmithKline and Novartis; and being a member of the Australian Technical Advisory Group on Immunisation and of the World Health Organization SAGE (Scientific Advisory Group of Experts) committee. Assistant Professor McVernon reported not having shares, paid employment or consultancies with CSL Limited; being an investigator on vaccine and epidemiological studies sponsored by CSL Limited and other companies; receiving support for conference attendance from a number of vaccine manufacturers; being a member of vaccine advisory boards for Novartis vaccines; and being a member of the Australian Technical Advisory Group on Immunisation. Professor Booy has received funding from CSL, Roche, Sanofi, GlaxoSmithKline and Wyeth/Pfizer to conduct sponsored research or attend and present at scientific meetings; any funding received is directed to a research account at the Children's Hospital at Westmead. Dr Leon Heron has no shares in pharmaceutical companies. His employer, the National Centre for Immunisation Research and Surveillance, The Children's Hospital at Westmead, has received funding from Novartis for consultancy work performed by Dr Heron. His employer has also received funding from GSK and Sanofi Pasteur for supporting Dr Heron's travel to conferences. His employer has also received funding for Dr Heron to be involved in research studies funded by Baxter, GSK, Pfizer, Merck, CSL, Roche, Novartis and Sanofi Pasteur. Assistant Professor Peter Richmond reported not having shares, or paid employment with CSL Limited; receiving travel assistance and an honorarium for a meeting of scientific experts to advise on the design and interpretation of studies of a CSL experimental influenza vaccine in the elderly in 2007; being an investigator on vaccine and epidemiological studies sponsored by CSL Limited and other companies; receiving support for conference attendance to present scientific data from Baxter, Sanofi Pasteur and Pfizer; and being a member of the Australian Technical Advisory Group on Immunisation. Dr Tony Walls reported not having shares, paid employment or consultancies with CSL Limited. He has received support for conference attendance from GlaxoSmithKline. Assistant Professor Marshall reported not having shares, paid employment or consultancies with CSL Limited; being an investigator on vaccine and epidemiological studies sponsored by CSL Limited and other companies; her employer receiving travel support from GlaxoSmithKline and CSL Limited for her to attend conferences to present independent scientific data; and being a member of vaccine advisory boards for GlaxoSmithKline, Wyeth/Pfizer and Novartis. Assistant Professor Reynolds reported not having shares, paid employment or consultancies with CSL Limited; being an investigator on vaccine and epidemiological studies sponsored by CSL Limited and other companies. Drs Hartel, Hu and Lai are employees of CSL Limited.
Supporting information
Table S1. Study group designation and vaccine schedule.
Table S2. Severity grading of solicited local and systemic adverse events.
Acknowledgements
We warmly thank the study participants and their families. Adelaide site: Chris Heath, Susan Lee, Sue Evans, Jan Walker, Rachel Chen, Diana Weber, Louise DeGaris, Michelle Clarke, Jane Tidswell, Mary Walker, Natalie Thomas, Kavita Rasiah. Brisbane site: Jacqui Langton, Aaron Buckner, Kylie Berglund, Dr Niall Conroy, Anne Cook, Irene Golin, Danielle de Plater, Ria Halstead, Sarah Spurr, Stacey van Dyk. Canberra site: Sandra Gillett, Dr Kaye Robertson. Melbourne site: Marita Kefford, Emily Bailey, Alice Holloway, Jacinta O'Keefe, Dr Maryanne Skeljo, Mairead Phelan, Dr Peter Howard, Dr Margie Danchin, Dr Karyn Alexander, Dr Lana Horng, Tamie Samyue, Michelle Boglis, Marie West, Paula Nathan, Annmarie McEvoy, Liz McGrath, Janet Briggs, Jane Ryrie, Clare Brophy, Ruth Lawrence, Judith Spotswood, Deb Saunders, Dr Nicole Rose, Dr Rebecca Taylor, Dr Sylvie Li Yim, Dr Jenny Davey, Dr Daniel Engelman, Dr Wei Lyn Fah. Perth site: Fiona McDonald, Sanela Bilic, Dr Gabriel Dixon, Shalene Nandlall, Dr Tanya Stoney, Dr Ushma Wadia. Sydney Children's Hospital site: Susan Smith, Meg Bruce, Vanessa Bonett. The Children's Hospital at Westmead site: Dr Kathrina Epino, Dr Gulam Khandaker, Dr Danform Lim, Dr Kunal Thacker, Pamela Cheung, Elizabeth Clarke, Elizabeth Deegan, Edwina Jacobs, Rosemary Joyce, Camille Lang, Jennifer Murphy, Laura Rost, Carol Shineberg. CSL: Dale P Cooper, Corrinne Clement, Susi Rogers, Daphne Sawlwin, Gail Dawson, Frank Albano.
Lambert et al (2013) Safety and tolerability of a 2009 trivalent inactivated split‐virion influenza vaccine in infants, children and adolescents. Influenza and Other Respiratory Viruses 7(5), 676–685
References
- 1. Lambert S, O'Grady KA, Gabriel S, Carter R, Nolan T. The cost of seasonal respiratory illnesses in Australian children: the dominance of patient and family costs and implications for vaccine use. Commun Dis Intell 2004; 28:510–516. [PubMed] [Google Scholar]
- 2. Iskander M, Booy R, Lambert S. The burden of influenza in children. Curr Opin Infect Dis 2007; 20:259–263. [DOI] [PubMed] [Google Scholar]
- 3. Lambert SB, Allen KM, Carter RC, Nolan TM. The cost of community‐managed viral respiratory illnesses in a cohort of healthy preschool‐aged children. Respir Res 2008; 9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoen AG, Buckeridge DL, Charland KML, Mandl KD, Quach C, Brownstein JS. Effect of expanded US recommendations for seasonal influenza vaccination: comparison of two pediatric emergency departments in the United States and Canada. CMAJ 2011; 183:E1025–E1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoberman A, Greenberg DP, Paradise JL. Effectiveness of inactivated influenza vaccine in preventing acute otitis media in young children: a randomized controlled trial. JAMA 2003; 290:1608–1616. [DOI] [PubMed] [Google Scholar]
- 6. Jansen AG, Sanders EA, Hoes AW, van Loon AM, Hak E. Effects of influenza plus pneumococcal conjugate vaccination versus influenza vaccination alone in preventing respiratory tract infections in children: a randomized, double‐blind, placebo‐controlled trial. J Pediatr 2008; 153:764–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Monto AS, Davenport FM, Napier JA, Francis T Jr. Modification of an outbreak of influenza in Tecumseh, Michigan by vaccination of schoolchildren. J Infect Dis 1970; 122:16–25. [DOI] [PubMed] [Google Scholar]
- 8. Hurwitz ES, Haber M, Chang A. Effectiveness of influenza vaccination of day care children in reducing influenza‐related morbidity among household contacts. JAMA 2000; 284:1677–1682. [DOI] [PubMed] [Google Scholar]
- 9. Reichert TA, Sugaya N, Fedson DS, Glezen WP, Simonsen L, Tashiro M. The Japanese experience with vaccinating schoolchildren against influenza. N Engl J Med 2001; 344:889–896. [DOI] [PubMed] [Google Scholar]
- 10. Piedra PA, Gaglani MJ, Kozinetz CA. Herd immunity in adults against influenza‐related illnesses with use of the trivalent‐live attenuated influenza vaccine (CAIV‐T) in children. Vaccine 2005; 23:1540–1548. [DOI] [PubMed] [Google Scholar]
- 11. Ghendon YZ, Kaira AN, Elshina GA. The effect of mass influenza immunization in children on the morbidity of the unvaccinated elderly. Epidemiol Infect 2006; 134:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davis MM, King JC Jr, Moag L, Cummings G, Magder LS. Countywide school‐based influenza immunization: direct and indirect impact on student absenteeism. Pediatrics 2008; 122:e260–e265. [DOI] [PubMed] [Google Scholar]
- 13. Kwong JC, Stukel TA, Lim J. The effect of universal influenza immunization on mortality and health care use. PLoS Med 2008; 5:e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention . Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010; 59:1–62. [PubMed] [Google Scholar]
- 15. Kelly H, Jacoby P, Dixon GA. Vaccine effectiveness against laboratory‐confirmed influenza in healthy young children: a case–control study. Pediatr Infect Dis J 2011; 30:107–111. [DOI] [PubMed] [Google Scholar]
- 16. Letter to General Practitioners – Update on Pandemic (H1N1) 2009 Influenza Vaccination. 2010. Available at http://www.flupandemic.gov.au/internet/panflu/publishing.nsf/Content/cmo-2010#12feb (Accessed 12 December 2012)
- 17. Jefferson T, Rivetti A, Harnden A, Di Pietrantonj C, Demicheli V. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev 2008; 8:CD004879. [DOI] [PubMed] [Google Scholar]
- 18. Nolan T, Richmond PC, McVernon J. Safety and immunogenicity of an inactivated thimerosal‐free influenza vaccine in infants and children. Influenza Other Respi Viruses 2009; 3:315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. A study to determine the immunogenicity and safety profile of CSL Limited's influenza virus vaccine compared to a US licensed comparator influenza virus vaccine in a pediatric population. Available at http://clinicaltrials.gov/ct2/show/results/NCT00959049 (Accessed 12 December 2012).
- 20. Whiley DM, Bialasiewicz S, Bletchly C. Detection of novel influenza A(H1N1) virus by real‐time RT‐PCR. J Clin Virol 2009; 45:203–204. [DOI] [PubMed] [Google Scholar]
- 21. Appuhamy RD, Beard FH, Phung HN, Selvey CE, Birrell FA, Culleton TH. The changing phases of pandemic (H1N1) 2009 in Queensland: an overview of public health actions and epidemiology. Med J Aust 2010; 192:94–97. [DOI] [PubMed] [Google Scholar]
- 22. Armstong PK, Dowse GK, Effler PV. Epidemiological study of severe febrile reactions in young children in Western Australia caused by a 2010 trivalent inactivated influenza vaccine. BMJ Open 2011; e000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clinical advice for immunisation providers on resumption of the use of 2010 trivalent seasonal vaccines in children less than 5 years of age, July 2010. 2010. Available at http://www.health.gov.au/internet/immunise/Publishing.nsf/content/immunise-atagi-statement-tiv (Accessed 12 December 2012).
- 24. Nolan T, McVernon J, Skeljo M. Immunogenicity of a monovalent 2009 influenza A(H1N1) vaccine in infants and children: a randomized trial. JAMA 2010; 303:37–46. [DOI] [PubMed] [Google Scholar]
- 25. A Clinical Trial of CSL's 2009 H1N1 Influenza Vaccine (CSL425) in a Healthy Pediatric Population in the USA. Available at http://clinicaltrials.gov/ct2/show/results/NCT00958243 (Accessed 12 December 2012).
- 26. Overview of vaccine regulation and safety monitoring and investigation into adverse events following 2010 seasonal influenza vaccination in young children. Attachment E: TGA influenza seasonal trivalent influenza vaccine laboratory investigation program. Available at http://www.tga.gov.au/safety/alerts-medicine-seasonal-flu-101008-atte.htm (Accessed 12 December 2012).
- 27. Kelly HA, Skowronski DM, De Serres G, Effler PV. Adverse events associated with 2010 CSL and other inactivated influenza vaccines. Med J Aust 2011; 195:318–320. [DOI] [PubMed] [Google Scholar]
- 28. Blyth CC, Currie AJ, Wiertsema SP. Trivalent influenza vaccine and febrile adverse events in Australia, 2010: clinical features and potential mechanisms. Vaccine 2011; 29:5107–5113. [DOI] [PubMed] [Google Scholar]
- 29. Maraskovsky E, Rockman S, Dyson A. Scientific investigations into febrile reactions observed in the paediatric population following vaccination with a 2010 Southern Hemisphere Trivalent Influenza Vaccine. Vaccine 2012; 30:7400–7406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Study group designation and vaccine schedule.
Table S2. Severity grading of solicited local and systemic adverse events.


