Fig. 4.
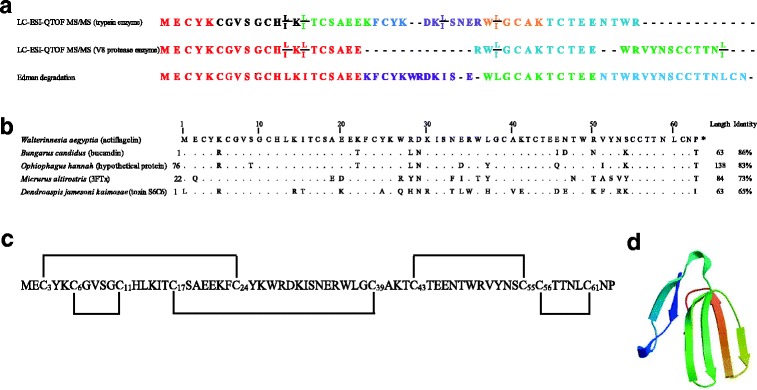
Actiflagelin amino acid sequence, disulfide bridge arrangement and putative structure. a Different sequences of actiflagelin that were obtained after MS/MS analyses of the reduced/alkylated/digested peptides and Edman degradation. In MS/MS de novo sequencing Ile and Leu residues cannot be resolved based on the CID activation mode and are therefore labelled (I/L on top of each other). b Sequence alignment of actiflagelin with homolog toxins retrieved from the protein BLAST. Hyphen-minus represents identical amino acid residues, and dots indicate the lack of residue at the position. The peptide lengths and percentages of sequence identities are given on the right. c Disulfide bridge organization of actiflagelin (in black) proposed by homology with bucandin. d SWISS-MODEL (http://swissmodel.expasy.org/) proposed a 3D-structure of actiflagelin
