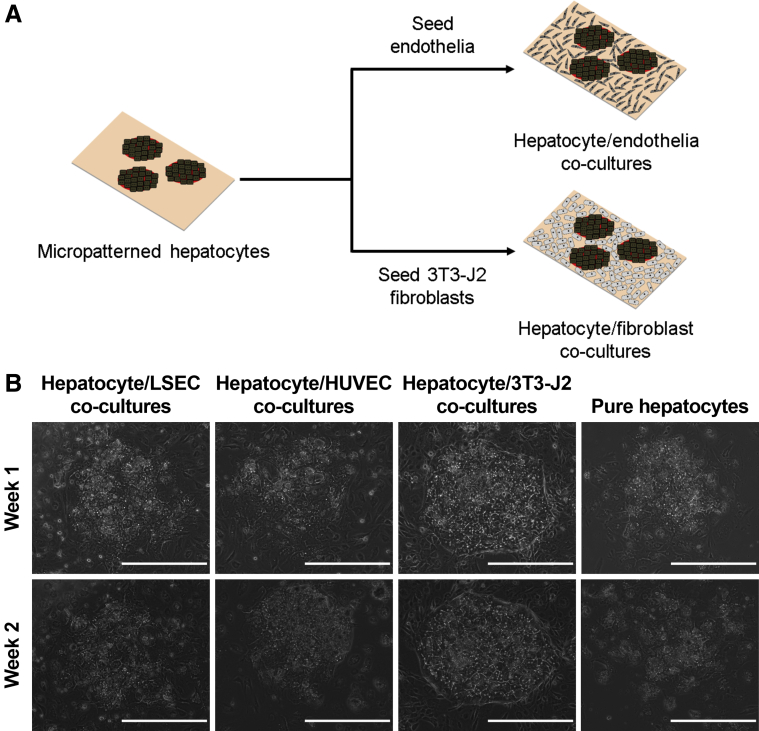
Abstract
Background and Aims
Modeling interactions between primary human hepatocytes (PHHs) and primary human liver sinusoidal endothelial cells (LSECs) in vitro can help elucidate human-specific mechanisms underlying liver physiology/disease and drug responses; however, existing hepatocyte/endothelial coculture models are suboptimal because of their use of rodent cells, cancerous cell lines, and/or nonliver endothelial cells. Hence, we sought to develop a platform that could maintain the long-term phenotype of PHHs and primary human LSECs.
Methods
Primary human LSECs or human umbilical vein endothelial cells as the nonliver control were cocultivated with micropatterned PHH colonies (to control homotypic interactions) followed by an assessment of PHH morphology and functions (albumin and urea secretion, and cytochrome P-450 2A6 and 3A4 enzyme activities) over 3 weeks. Endothelial phenotype was assessed via gene expression patterns and scanning electron microscopy to visualize fenestrations. Hepatic responses in PHH/endothelial cocultures were benchmarked against responses in previously developed PHH/3T3-J2 fibroblast cocultures. Finally, PHH/fibroblast/endothelial cell tricultures were created and characterized as described previously.
Results
LSECs, but not human umbilical vein endothelial cells, induced PHH albumin secretion for ∼11 days; however, neither endothelial cell type could maintain PHH morphology and functions to the same magnitude/longevity as the fibroblasts. In contrast, both PHHs and endothelial cells displayed stable phenotype for 3 weeks in PHH/fibroblast/endothelial cell tricultures; furthermore, layered tricultures in which PHHs and endothelial cells were separated by a protein gel to mimic the space of Disse displayed similar functional levels as the coplanar tricultures.
Conclusions
PHH/fibroblast/endothelial tricultures constitute a robust platform to elucidate reciprocal interactions between PHHs and endothelial cells in physiology, disease, and after drug exposure.
Keywords: Micropatterned Cocultures, Tricultures, LSECs, HUVECs, 3T3-J2 Fibroblasts
Abbreviations used in this paper: CD31, cluster of differentiation 31; CD54, cluster of differentiation 54; cDNA, complementary DNA; CYP450, cytochrome P-450; ECM, extracellular matrix; F8, factor VIII; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HUVECs, human umbilical vein endothelial cells; LSECs, liver sinusoidal endothelial cells; NPCs, nonparenchymal cells; PHHs, primary human hepatocytes; SEM, scanning electron microscope; vWF, von Willebrand factor
Graphical abstract
Summary.
We show that in contrast to functionally declining cocultures containing primary human hepatocytes and primary human liver sinusoidal endothelial cells, tricultures containing primary human hepatocytes, 3T3-J2 fibroblasts, and liver sinusoidal endothelial cells display stable hepatic and endothelial phenotypes for 3 weeks in vitro.
The parenchymal hepatocytes in the liver interact with liver sinusoidal endothelial cells (LSECs), which comprise ∼70% of the total nonparenchymal cell (NPC) types in the liver.1 Separated from the hepatocytes by the space of Disse, a thin layer of extracellular matrix (ECM) proteins, LSECs contain numerous fenestrations arranged in sieve plates and play important roles in liver biology. For instance, LSECs clear waste molecules (including viruses) entering portal venous blood1; are important for induction of CD8+ T-cell tolerance2; synthesize the critical coagulation cofactor, factor VIII3; contribute to liver regeneration via increased production of cytokines (eg, hepatocyte growth factor)4; release exosomes that modulate antiviral activity in hepatitis C virus–infected hepatocytes in coculture5; can become insulin resistant and defenestrated in nonalcoholic fatty liver disease6; and experience toxicity to certain drugs.7 Therefore, developing novel liver models containing LSECs and hepatocytes can aid in drug discovery efforts for liver diseases and enable a better understanding of how reciprocal LSEC-hepatocyte interactions modulate cellular phenotypes in liver physiology.
Significant species-specific differences in drug metabolism and other liver pathways8, 9, 10 necessitate the supplementation of animal data with human-relevant in vitro assays for drug development.11 Given their physiological relevance, isolated primary human hepatocytes (PHHs) are widely considered to be ideal for building human liver models. However, when cultured in the presence of ECM proteins (eg, collagen) alone, PHHs rapidly (hours to days) decline in critical phenotypic functions, such as cytochrome P-450 (CYP450) enzyme activities,12 insulin responsiveness,13 and expression of the master liver transcription factor, hepatocyte nuclear factor 4α.14 Similarly, when cultured alone, LSECs lose their characteristic fenestrations and undergo apoptosis within a few days.15 In contrast to hepatocyte monocultures, coculture with both liver- and nonliver-derived NPC types can enhance hepatocyte functions in culture.16 Endothelial cells have been previously explored toward transiently enhancing hepatocyte functions in cocultures relative to declining hepatocyte monocultures. However, many such hepatocyte-endothelial coculture studies use rodent cells17, 18, 19, 20, 21 that do not completely suffice for modeling human liver biology. Furthermore, the use of abnormal cancerous cell lines22, 23, 24 and/or nonliver endothelial cells17, 19, 21, 25 may provide a first approximation of hepatocyte-endothelial interactions but needs to be complemented with the use of primary cells from human liver tissue to determine similarities and differences in observed cell responses. Indeed, the Yarmush group has created in vitro cocultures of PHHs and primary human LSECs, which showed high level of low-density lipoprotein uptake in PHHs in the presence of LSECs,26 and increased (∼1.3-fold) hepatic CYP1A activity in serum-free coculture with endothelial cells under high (95%) oxygen levels.27 However, it is not clear from these short-term (≤24 hours) data sets whether LSECs can induce high levels of phenotypic functions in PHHs over long-term (weeks) culture as compared with PHH monocultures. Additionally, the differential effects of LSECs on PHH functions relative to nonliver vascular endothelial cells remain to be elucidated.
To address the limitations with the previously mentioned hepatocyte-endothelial coculture studies, here we sought to first elucidate the effects of primary human LSECs on the long-term functions of PHHs with comparisons to nonliver endothelial cells (human umbilical vein endothelial cells [HUVECs]) and PHH monocultures. We benchmarked the effects of endothelial cells on PHHs to the effects of 3T3-J2 murine embryonic fibroblasts, a cell type that expresses hepatocyte-supporting molecules present in the liver28 and is known to induce high levels of functions in PHHs for 4–6 weeks in vitro.12 To maintain consistent homotypic PHH contacts/interactions across the various coculture configurations toward isolating the effects of the heterotypic interactions between PHHs and NPCs, we used a previously developed semiconductor-driven micropatterning technique to create so-called micropatterned cocultures in which PHHs are first clustered onto circular collagen-coated domains of precise/reproducible diameters and subsequently surrounded by an NPC layer containing 1 or more cell types.29 Lastly, we determined PHH/fibroblast/endothelial triculture configurations, including those in which the LSECs were separated from PHHs via an ECM protein gel as in the space of Disse in vivo, which promoted stable PHH and LSEC phenotype over several weeks toward developing a model system that has utility in drug development and to better understand PHH and LSEC interactions in liver physiology and disease.
Materials and Methods
Endothelial Culture
Primary human LSECs5 and TMNK immortalized liver endothelial cells30 were obtained from Dr. Hugo Rosen of the University of Colorado-Denver School of Medicine. A second donor of LSECs was purchased from ScienCell (Carlsbad, CA) and used to verify the trends observed with the first LSEC donor. Pooled-donor HUVECs were purchased from Lonza (Williamsport, PA). Primary endothelial cells were cultured at 37ºC, 10% CO2 in EGM-2 BulletKit medium (Lonza) on tissue culture polystyrene coated with 2 μg/cm2 fibronectin (Corning Life Sciences, Manassas, VA). TMNK cells were cultured at 37ºC, 10% CO2 in a high-glucose Dulbecco’s modified eagle medium base (Corning) supplemented with 10% (vol/vol) bovine serum and 1% (vol/vol) penicillin-streptomycin. Primary endothelial cell types were passaged no more than 6 times; before coculture with PHHs as described later, the endothelial cells were treated with 0.05% (m/vol) trypsin for 5 minutes to release cells into suspension, centrifuged, and resuspended in fresh culture medium.
Micropatterned Coculture and Triculture Fabrication
Cryopreserved PHHs were commercially obtained from Triangle Research Labs (Durham, NC); donors included HUM4011 (26-year-old white male who died of cardiac arrest) and HUM4055A (54-year-old white female who died of stroke). PHHs were thawed, counted, and viability was assessed as previously described.31 PHHs were subsequently micropatterned on collagen-coated domains as previously described.29 Briefly, adsorbed rat tail collagen I (Corning) was lithographically patterned in each well of a 24-well plate to create 500-μm diameter circular domains spaced 1200 μm apart, center-to-center. PHHs selectively attached to the collagen domains leaving ∼30,000 attached PHHs on ∼90 collagen-coated islands within each well of a 24-well plate. The next day after adherent PHHs had spread to fill in the collagen domains, the cultures were incubated with fibronectin (2 μg/cm2) to coat the remaining surface area with ECM protein to enable attachment of NPC types, which included either 3T3-J2 murine embryonic fibroblasts or endothelial cells at ∼90,000 per well; the endothelial cells included were either HUVECs or primary human LSECs.
To create coplanar micropatterned tricultures, ∼6000 endothelial cells (HUVECs or LSECs) were mixed into a suspension of ∼84,000 3T3-J2 fibroblasts, and then this mixture was seeded onto micropatterned PHH colonies (∼30,000 total PHHs) in each well of a 24-well plate. The 1:5 endothelial/PHH ratio corresponds to the approximate ratio in the liver.32 To create layered micropatterned tricultures, micropatterned cocultures containing PHH colonies surrounded by the fibroblasts were first overlaid with a thin gel (250 μg/mL) of Matrigel (Corning) and then ∼6000 endothelial cells were seeded on top of the Matrigel the following day. Culture medium containing 40 ng/mL recombinant vascular endothelial growth factor15 (Thermo Fisher Scientific, Waltham, MA) in a high-glucose Dulbecco’s modified eagle medium base (Corning) was replaced on cocultures and tricultures every 2 days (300 μL/well). Other culture medium components were described previously.31
Gene Expression Analysis
Total RNA was isolated, purified, and reverse transcribed into complementary DNA (cDNA) as previously described.31 Briefly, RNA was extracted from the cultures with the RNeasy kit (Qiagen, Germantown, MD) and homogenized via centrifugation through homogenizing columns (Omega Bio-Tek, Norcross, GA). Genomic DNA was removed with a 1-hour treatment with DNAse I (New England Biolabs, Ipswich, MA), and cDNA was synthesized with the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Then, 250 ng of cDNA was added to each quantitative polymerase chain reaction along with the Taqman master mix (Thermo Fisher Scientific) and predesigned Taqman human-specific primer/probe sets per manufacturer’s protocols; primer/probe sets were selected for endothelial genes including cluster of differentiation 31 (CD31), cluster of differentiation 54 (CD54), factor VIII (F8), and von Willebrand factor (vWF). The primer/probe sets were selected to be human-specific without cross-reactivity to 3T3-J2 mouse DNA; however, Taqman primer/probe sequences are proprietary to the manufacturer. Gene expression was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Cell Morphologic and Functional Assessments
Cell morphology was monitored using an EVOS FL microscope (Thermo Fisher Scientific) with standard 4×, 10×, and 20× phase contrast objectives. PHH projected area from phase contrast pictures was quantified using ImageJ software.33 Concentrations of human albumin and urea in collected cell culture supernatants were assayed using previously published protocols.31 Briefly, albumin secretions were assessed using a competitive enzyme-linked immunosorbent assay, whereas urea production was measured via a colorimetric reaction with diacetyl monoxime, acid, and heat (kit from Stanbio Labs, Boerne, TX). CYP450 enzyme activities were measured by first incubating cultures in substrates for 1 hour at 37°C and then detecting either the luminescence or fluorescence of metabolites using previously described protocols.31 CYP2A6 activity was measured by the modification of coumarin to fluorescent 7-hydroxycoumarin (Sigma-Aldrich, St. Louis, MO), and CYP3A4 activity was measured by cleavage of luciferin-IPA into luminescent luciferin (Promega, Madison, WI). Live cultures containing PHHs were incubated with 2 μg/mL 5 (and 6)-carboxy-2′,7′-dichlorofluorescein diacetate for 10 minutes in serum-free and phenol-free culture medium at 37°C, washed 3 times in serum-free and phenol-free culture medium, and imaged using the green fluorescent protein (470 nm excitation, 510 nm emission) light cube on the microscope to visualize functional bile canaliculi.
Scanning Electron Microscopy
Cultures were fixed in glutaraldehyde (Sigma-Aldrich) diluted to 2.5% (vol/vol) in phosphate-buffered saline (1X PBS, Corning) solution for 20 minutes at room temperature, and fixed cell cultures were washed 3 times with 1X PBS. Then, fixed samples were dehydrated through a series of washes using molecular-grade ethanol (Fisher Scientific, Pittsburgh, PA) to dry the samples thoroughly without damaging the cellular structures; washes were performed for 10 minutes each at 35%, 50%, 75%, 85%, 95%, 100%, and 100% (vol/vol) ethanol. Following the final ethanol wash, cultures were dried chemically with hexamethyldisilizane (Alfa Aesar) for 10 minutes, excess hexamethyldisilizane was removed, and samples were air dried for 10 minutes at room temperature. Prepared samples were secured to a specimen mount using carbon adhesive tabs. A scanning electron microscope (SEM) coating unit E5100 Series II (Polaron Instruments, London, England) was used to sputter coat samples with a 4.125-nm-thick gold/palladium layer, and samples were then imaged using a Hitachi S-3000 variable pressure SEM (Tokyo, Japan).
Data Analysis
Each experiment was carried out in triplicate wells for each condition. Studies were repeated in 2 PHH and 2 LSEC donors to confirm trends. Data processing and visualization were performed using Microsoft Excel and GraphPad Prism (La Jolla, CA). Gene expression data were calculated as fold changes with respect to pure LSECs using the ΔΔCT method with GAPDH as the housekeeping gene. Statistical significance was determined with a 1- or 2-way analysis of variance followed by a Bonferroni pair-wise post hoc test (P < .05).
Results
Comparison of Primary Human Hepatocytes/Endothelial and Primary Human Hepatocytes/Fibroblast Cocultures
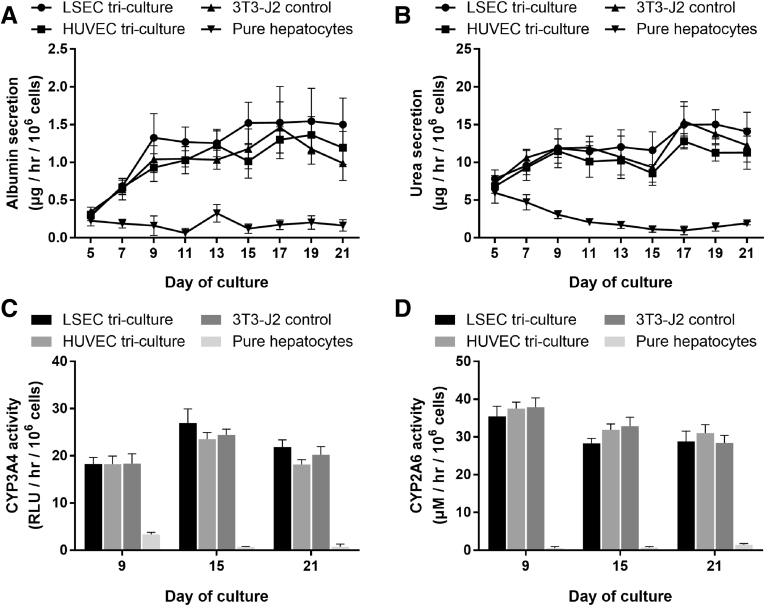
Primary human LSECs and primary HUVECs displayed prototypical endothelial morphology for up to 6 passages in vitro (Figure 1) and could be subsequently used for cocultivation with PHHs. Micropatterned cocultures of PHHs and endothelial cells (either LSECs or HUVECs) were compared with cocultures of PHHs and 3T3-J2 fibroblasts (Figure 2A); in such cocultures, 30,000 PHHs were surrounded by 90,000 NPCs. Both PHH/LSEC and PHH/HUVEC cocultures displayed a loss of prototypical hepatic morphology (ie, polygonal shape, multinucleation, and visible bile canaliculi) over 2 weeks (Figure 2B). In contrast, PHH/fibroblast cocultures maintained hepatic morphology over at least 2 weeks. Micropatterned PHH monocultures spread out (dedifferentiated) as expected from a previous study.12
Figure 1.
Morphology of pure endothelial cells during expansion. Before coculture with PHHs, endothelial cells were expanded in tissue culture flasks as pure cultures up to 6 passages. Both primary human LSECs (A, C) and HUVECs (B, D) displayed similar morphologic characteristics. (A, B) Scale bars = 1000 μm; (C, D) Scale bars = 400 μm.
Figure 2.
Morphology of PHH/endothelial cell and PHH/3T3-J2 fibroblast cocultures relative to PHH monocultures. (A) Schematic depicting the creation of the coculture models. PHHs were micropatterned using soft-lithography to control for homotypic interactions and then surrounded by NPCs as described previously.41 (B) Morphology of different coculture models over the course of 2 weeks in comparison with the pure PHH monocultures (right). Note the prototypical PHH morphology (ie, polygonal shape, multinucleation, and presence of visible bile canaliculi) in the PHH/fibroblast cocultures and spread-out (dedifferentiated) morphology in the PHH monocultures. All scale bars = 400 μm.
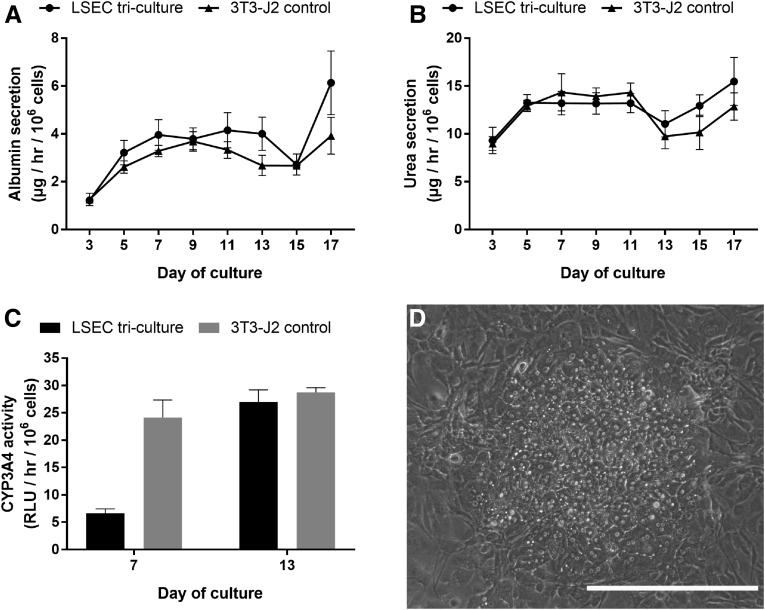
At the functional level, PHH/fibroblast cocultures outperformed PHH/endothelial cell cocultures. Albumin (Figure 3A) and urea secretions (Figure 3B), CYP3A4 activity (Figure 3C), and CYP2A6 activity (Figure 3D) in PHH/endothelial cell cocultures had steady-state values that were ∼5%–20% of the values in PHH/fibroblast cocultures. The PHH/fibroblast cocultures maintained stable levels of all measured functions over 3 weeks, whereas most functions in PHH/endothelial cell cocultures declined over time. Modulating the PHH:endothelial ratio to 5:1 (physiologic32) or 1:4 in cocultures did not rescue the PHH phenotype (data not shown). Furthermore, cocultures created using a second donor of LSECs followed similar trends in albumin secretions, urea secretions, CYP3A4 activity, and morphology (Figure 4). Nonetheless, PHH/LSEC cocultures had statistically higher albumin secretions than PHH/HUVEC cocultures (∼3.7-fold on Day 7 and ∼10-fold on Day 11) and PHH monocultures (undetectable levels) until 11 days in culture, whereas urea secretion and CYP450 enzyme activities were statistically similar (Figure 3). Finally, immortalized human liver endothelial cells (TMNK line), previously used for coculture with PHHs,34 were also not able to stabilize PHH morphology or functions (Figure 5).
Figure 3.
Hepatic functions in PHH/endothelial cell and PHH/3T3-J2 fibroblast cocultures relative to PHH monocultures. Cocultures and PHH monocultures were created as depicted in Figure 2A (all culture models shown contained micropatterned PHHs) followed by an assessment of hepatic functions over time, including albumin secretion (A), urea secretion (B), CYP3A4 enzyme activity (C), and CYP2A6 enzyme activity (as measured by the production of 7-HC) (D). Error bars represent standard deviations (n = 3 wells). **P < .01 and ***P < .001 between the PHH/LSEC cocultures and PHH/HUVEC cocultures or PHH monocultures.
Figure 4.
PHH/endothelial cell cocultures created using a second primary human LSEC donor relative to PHH/fibroblast control cocultures. Cocultures were created as depicted in Figure 2A (all culture models shown contained micropatterned PHHs) followed by an assessment of hepatic functions over time, including albumin secretion (A), urea secretion (B), and CYP3A4 enzyme activity (C). Error bars represent standard deviations (n = 3 wells). PHH morphology in PHH/LSEC cocultures after 1 week is shown in (D), and PHH morphology in PHH/fibroblast cocultures is shown in Figure 2B. Scale bar = 400 μm.
Figure 5.
PHH/endothelial cell cocultures created using the immortalized human liver endothelial cell line (TMNK) relative to PHH/fibroblast control cocultures. Cocultures were created as depicted in Figure 2A (all culture models shown contained micropatterned PHHs) followed by an assessment of PHH morphology in PHH/TMNK cocultures after 1 week (A) and 2 weeks (B). PHH morphology in PHH/fibroblast cocultures is shown in Figure 2B. Scale bar = 400 μm. Albumin (C) and urea (D) secretions were also measured from the PHH/TMNK and PHH/fibroblast cocultures over time. Error bars represent standard deviations (n = 3 wells).
The effects of endothelial cells on PHH functions in cocultures were observed irrespective of the culture medium formulation because subjecting the PHH/endothelial cocultures to endothelial culture medium (as that used for expanding pure endothelial cells) or hepatocyte culture medium or a 1:1 mixture of the 2 types of media did not enable long-term rescue of the PHH phenotype (data not shown); thus, supplementing the hepatocyte medium with vascular endothelial growth factor, a previously developed hepatocyte-endothelial culture medium,15 was used for the remainder of the studies. Therefore, LSECs, but not HUVECs, can induce transient and statistically significant albumin secretion in PHHs for ∼11 days; however, neither endothelial cell type can induce functions to the same magnitude and stability as the 3T3-J2 fibroblasts irrespective of PHH/endothelial ratio and medium formulation.
Hepatic Phenotype in Coplanar Primary Human Hepatocytes/Fibroblast/Endothelial Tricultures
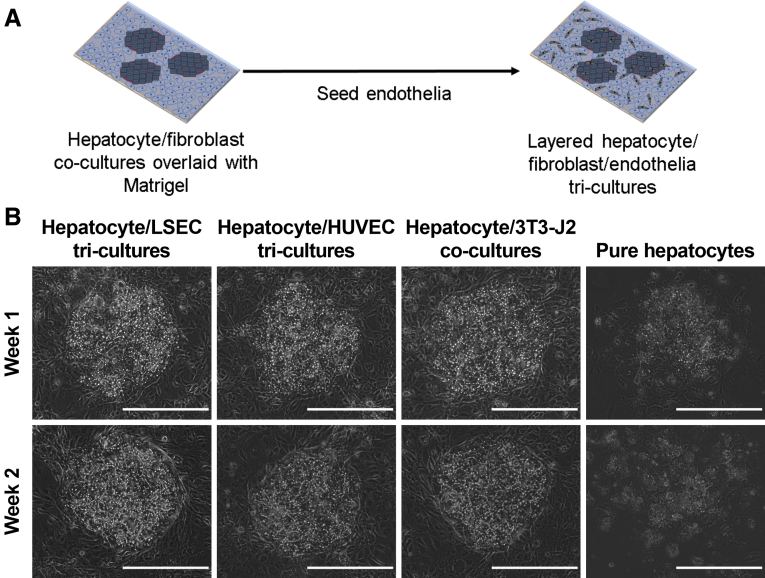
As opposed to PHH/endothelial cocultures, PHH/fibroblast/endothelial tricultures were seeded as shown in Figure 6A. Briefly, after PHHs attached and spread on micropatterned collagen-coated domains, a mixture of endothelial cells (LSECs or HUVECs) and 3T3-J2 fibroblasts was seeded and filled the remaining area around the PHH colonies. A PHH/endothelial cell ratio of 5:1 was selected to match that observed in vivo. Over the course of 3 weeks, PHH/fibroblast/LSEC and PHH/fibroblast/HUVEC tricultures displayed a hepatic morphology comparable with that observed in PHH/fibroblast cocultures with respect to multinucleation, polygonal shape, and the presence of visible bile canaliculi between hepatocytes (Figure 6B).
Figure 6.
Morphology of PHH/fibroblast/endothelial cell tricultures (containing either LSECs or HUVECs) relative to PHH/3T3-J2 fibroblast control cocultures and PHH monocultures. (A) Schematic depicting the creation of the tricultures; cocultures were created as depicted in Figure 2A. (B) Morphology of tricultures models over the course of 2 weeks in comparison with the PHH/fibroblast cocultures and pure PHH monocultures. Note the prototypical PHH morphology (ie, polygonal shape, multinucleation, and presence of visible bile canaliculi) in the tricultures/cocultures and spread-out (dedifferentiated) morphology in the PHH monocultures. All scale bars = 400 μm.
At the functional level, when treated with the bile canaliculi stain, 5 (and 6)-carboxy-2′,7′-dichlorofluorescein diacetate (substrate for transporters, multidrug resistance-associated proteins 2/335), adjacent PHHs in the tricultures displayed functional bile canaliculi as in the PHH/fibroblast cocultures, whereas PHH monocultures displayed little to no functional bile canaliculi (Figure 7). Furthermore, PHH/fibroblast/endothelial cell tricultures secreted albumin (Figure 8A) and urea (Figure 8B) at steady-state levels of ∼1.4 μg/h/106 cells and ∼12 μg/h/106 cells, respectively, for 3 weeks; these secretion levels were statistically similar to the levels measured in PHH/fibroblast cocultures. Similarly, CYP3A4 (Figure 8C) and CYP2A6 (Figure 8D) enzyme activities were statistically similar across the tricultures and PHH/fibroblast cocultures. However, PHH monocultures displayed a severe decline in functions (Figure 8). Furthermore, the previously mentioned morphologic and functional trends in tricultures relative to cocultures were also observed with a second primary LSEC donor (Figure 9). However, in contrast to primary human LSECs and primary HUVECs, the use of the immortalized TMNK line in tricultures with fibroblasts did not lead to stable PHH morphology or functions (Figure 10), likely because of overgrowth of the TMNK cells.
Figure 7.
Bile canaliculi staining in PHH/fibroblast/endothelial cell tricultures (containing either LSECs or HUVECs) relative to PHH/3T3-J2 fibroblast control cocultures and PHH monocultures. Cocultures and tricultures were created as depicted in Figures 2A and 6A (all culture models shown contained micropatterned PHHs), respectively, followed by an assessment of functional bile canaliculi (green stain) after 2 weeks as described in the Methods. Both PHH/fibroblast/LSEC tricultures (A) and PHH/fibroblast/HUVEC tricultures (B) contained active bile canaliculi as in PHH/fibroblast cocultures (C). However, pure PHH monocultures showed no noticeable bile canaliculi (D). The bile canaliculi stain is overlaid on phase contrast image for each culture model. All scale bars = 400 μm.
Figure 8.
Hepatic functions in PHH/fibroblast/endothelial cell tricultures (containing either LSECs or HUVECs) relative to PHH/3T3-J2 fibroblast control cocultures and PHH monocultures. Cocultures and tricultures were created as depicted in Figures 2A and 6A (all culture models shown contained micropatterned PHHs), respectively, followed by an assessment of hepatic functions over time, including albumin secretion (A), urea secretion (B), CYP3A4 enzyme activity (C), and CYP2A6 enzyme activity (as measured by the production of 7-HC) (D). Error bars represent standard deviations (n = 3 wells).
Figure 9.
PHH/fibroblast/endothelial cell tricultures creating using a second primary human LSEC donor relative to PHH/fibroblast control cocultures. Cocultures and tricultures were created as depicted in Figures 2A and 6A, respectively, followed by an assessment of hepatic functions over time, including albumin secretion (A), urea secretion (B), and CYP3A4 enzyme activity (C). Error bars represent standard deviations (n = 3 wells). PHH morphology in PHH/fibroblast/LSEC tricultures after 1 week is shown in (D), and PHH morphology in PHH/fibroblast cocultures is shown in Figure 2B. Scale bar = 400 μm.
Figure 10.
PHH/fibroblast/endothelial cell tricultures created using the immortalized human liver endothelial cell line (TMNK) relative to PHH/fibroblast control cocultures. Cocultures and tricultures were created as depicted in Figures 2A and 6A, respectively, followed by an assessment of PHH morphology in PHH/fibroblast/TMNK tricultures after 1 week (A) and 2 weeks (B). PHH morphology in PHH/fibroblast cocultures is shown in Figure 2B. Scale bar = 400 μm. Albumin (C) and urea (D) secretions were also measured from the PHH/fibroblast/TMNK tricultures and PHH/fibroblast control cocultures over time. Error bars represent standard deviations (n = 3 wells).
Endothelial Phenotype in Coplanar Primary Human Hepatocytes/Fibroblast/Endothelial Tricultures
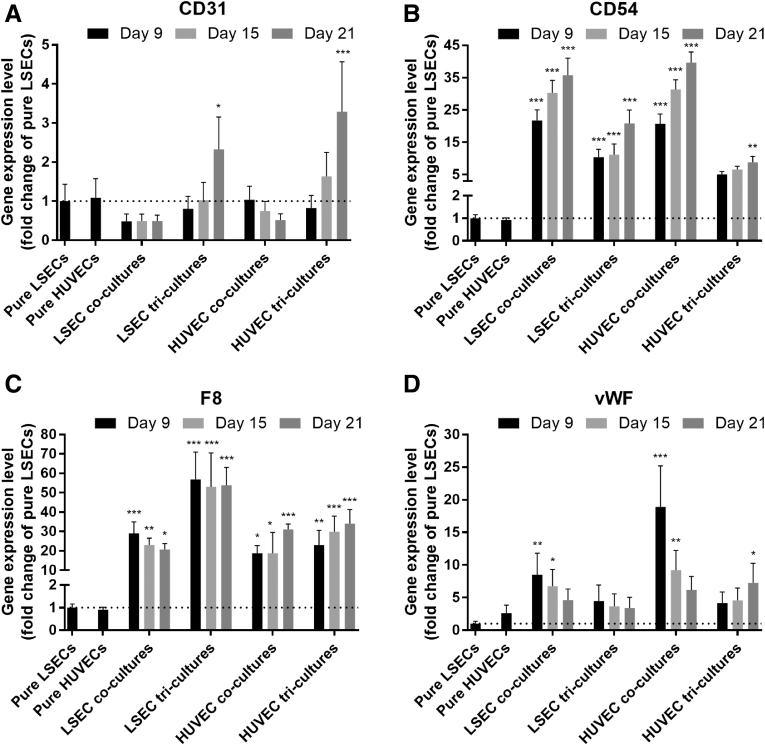
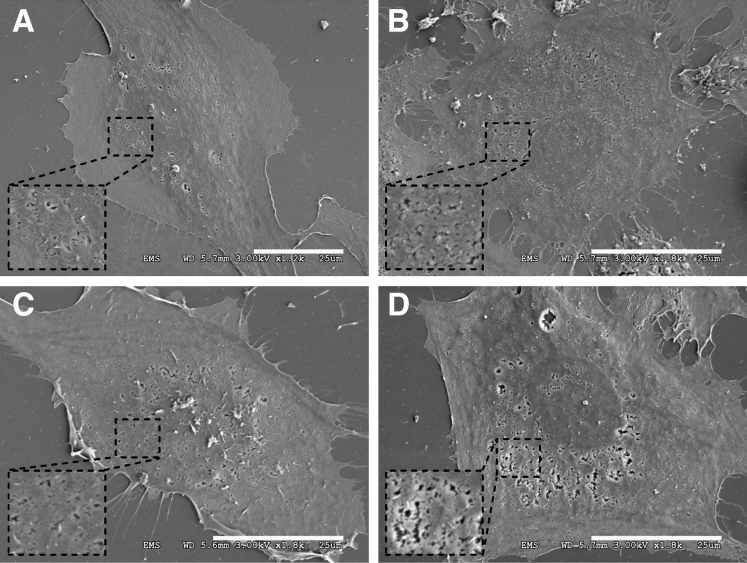
The presence of endothelial cells in tricultures over time was confirmed via gene expression analysis. Specifically, CD31 (Figure 11A) and CD54 (Figure 11B) gene expression levels increased in PHH/fibroblast/endothelial cell tricultures, whereas F8 (Figure 11C) and vWF (Figure 11D) expression levels were relatively stable in tricultures over the course of 3 weeks. In PHH/endothelial cell cocultures, all the previously mentioned gene expression markers were detected over 3 weeks; however, similarities and differences with the tricultures were observed. CD31 gene expression in PHH/endothelial cell cocultures was generally lower than tricultures over 3 weeks, CD54 gene expression in cocultures was higher than tricultures, F8 gene expression in PHH/LSEC cocultures was lower than PHH/fibroblast/LSEC tricultures but similar between PHH/HUVEC cocultures and PHH/fibroblast/HUVEC tricultures, and vWF expression in cocultures was higher than tricultures. We also verified using SEM the presence of fenestrations on LSECs in pure cultures (Figure 12A), when cocultured with 3T3-J2 fibroblasts but without PHHs (Figure 12B), in PHH/LSEC cocultures (Figure 12C), and in PHH/fibroblast/LSEC tricultures (Figure 12D). LSECs displayed noticeable fenestrations regardless of the culture format. The previously mentioned endothelial characterization results show that both LSECs and HUVECs display prototypical markers over several weeks in both cocultures and triculture configurations; however, the PHH phenotype is enhanced only in the presence of the fibroblasts in cocultures and tricultures.
Figure 11.
Endothelial gene expression patterns in different culture models. PHH/endothelial cell cocultures and PHH/fibroblast/endothelial cell tricultures were created as depicted in Figures 2A and 6A, respectively, followed by evaluation of endothelial (HUVECs or LSECs) gene expression patterns over time using reverse-transcription quantitative polymerase chain reaction, including CD31 (A), CD54 (B), F8 (C), and vWF (D). Data plotted are fold changes with respect to pure LSECs calculated with the ΔΔCT method using GAPDH as the housekeeping gene. Error bars represent standard deviations (n = 3 wells). *P < .05, **P < .01, and ***P < .001 between the coculture or triculture condition relative to pure LSECs.
Figure 12.
Visualization of fenestrations via SEM in primary human LSECs in different culture models. Pure LSEC cultures (A), LSECs cocultured with 3T3-J2 fibroblasts (B), PHH/LSEC cocultures (C), and PHH/fibroblast/LSEC tricultures (D) each display noticeable fenestrations. All scale bars = 25 μm.
Characterization of Layered Primary Human Hepatocytes/Fibroblast/Endothelial Tricultures
To mimic the space of Disse, we created a layered version of the triculture model by overlaying a PHH/fibroblast coculture with a thin gel (250 μg/mL) of Matrigel and subsequently seeding endothelial cells (LSECs or HUVECs) on top of the gel (Figure 13A). As in the PHH/fibroblast/endothelial cell coplanar triculture model, PHHs in the layered configuration maintained prototypical hepatocyte morphology with polygonal shape, multinucleation, and visible bile canaliculi between adjacent PHHs (Figure 13B). However, micropatterned PHHs overlaid with Matrigel but without any NPC types deteriorated in morphology.
Figure 13.
Morphology of layered PHH/fibroblast/endothelial cell tricultures (containing either LSECs or HUVECs) relative to PHH/3T3-J2 fibroblast control cocultures and PHH monocultures. (A) Schematic depicting the creation of the layered tricultures; PHH/fibroblast control cocultures and PHH monocultures were created as depicted in Figure 2A and subsequently overlaid with Matrigel to account for the effects of the protein gel on PHH functions. (B) Morphology of layered tricultures models over the course of 2 weeks in comparison with the Matrigel-coated PHH/fibroblast cocultures and Matrigel-coated pure PHH monocultures. Note the prototypical PHH morphology (ie, polygonal shape, multinucleation, and presence of visible bile canaliculi) in the tricultures/cocultures and spread-out (dedifferentiated) morphology in the PHH monocultures. All scale bars = 400 μm.
At the functional level, PHH/fibroblast/endothelial cell layered tricultures secreted albumin (Figure 14A) and urea (Figure 14B) at steady-state levels of ∼1.4 μg/h/106 cells and ∼12 μg/h/106 cells, respectively, for 3 weeks; these secretion levels were statistically similar to the levels measured in control PHH/fibroblast cocultures with a Matrigel overlay. Similarly, CYP3A4 (Figure 14C) and CYP2A6 (Figure 14D) enzyme activities were ∼20 RLU/h/106 cells and ∼32 μM 7-hydroxycoumarin/h/106 cells for both PHH/fibroblast/endothelial cell layered tricultures and PHH/fibroblast cocultures with a Matrigel overlay. Overall, the layered tricultures were highly similar in hepatic morphology and functional levels over several weeks as the coplanar tricultures, suggesting that separating the endothelial cells from PHHs did not lead to higher induction of hepatic phenotype (compare Figures 6 and 8 with Figures 13 and 14).
Figure 14.
Hepatic functions in layered PHH/fibroblast/endothelial cell tricultures (containing either LSECs or HUVECs) relative to PHH/3T3-J2 fibroblast control cocultures and PHH monocultures. Matrigel-coated tricultures and cocultures were created as described in Figure 13, followed by an assessment of hepatic functions over time, including albumin secretion (A), urea secretion (B), CYP3A4 enzyme activity (C), and CYP2A6 enzyme activity (as measured by the production of 7-HC) (D). Error bars represent standard deviations (n = 3 wells). *P < .05, **P < .01, and ***P < .001 between the PHH/fibroblast/LSEC tricultures and PHH/fibroblast cocultures.
Comparison of Primary Human Hepatocytes Projected Surface Area in Various Culture Formats
To confirm qualitative observations of PHH spreading out or lack thereof in various culture formats, we quantified the projected surface area of PHHs cultured in all models developed here (Figure 15). PHHs in monocultures or in PHH/endothelial cocultures tended to spread out and occupy more projected surface area. However, PHHs in cultures containing 3T3-J2 fibroblasts (PHH/fibroblast cocultures, PHH/fibroblast/endothelial coplanar tricultures, or PHH/fibroblast/endothelial layered tricultures) maintained their polygonal shape and occupied less projected surface area.
Figure 15.
Quantification of projected PHH surface area in various culture formats. Cocultures, tricultures, and layered tricultures were created as illustrated in Figures 2A, 6A, and 13A, respectively. After a week of culture, 3 PHHs from each of 3 independent islands of each condition were quantified for their projected cellular area. Cultures with (A) LSECs and (B) HUVECs both maintained smaller hepatocyte area when cultured in PHH/fibroblast/endothelial cell tricultures (with or without a Matrigel overlay) as compared with PHH/endothelial cell cocultures. Error bars represent standard deviations (n = 9 hepatocytes per condition). **P < .01 and ***P < .001 between the indicated culture formats.
Discussion
In this study, we developed the first-of-its-kind cell culture platform that induces high and stable levels of phenotypic functions in both PHHs and primary human LSECs over the course of several weeks. We initially created cocultures of PHHs and LSECs, while using HUVECs as the nonliver-endothelial cell control; micropatterning was used to control PHH homotypic interactions toward isolating the effects of PHH/NPC interactions.29 Fibronectin was used here for enabling endothelial cell attachment because this ECM protein has been shown previously to facilitate the attachment of LSECs.15 However, fibronectin is not amenable to the plasma ablation micropatterning technique used here (data not shown). Therefore, we used adsorbed collagen I for creating micropatterned PHH colonies as in previous studies29, 36; the use of collagen I for PHH attachment is also widespread in the field of PHH culture.11 Hepatic albumin secretion was statistically higher in PHH/LSECs cocultures for ∼11 days than in PHH/HUVEC cocultures and PHH monocultures. However, the effects of both endothelial cell types on PHH phenotype were transient and functions declined over time irrespective of PHH-to-endothelial ratios and medium formulations tested.
Our observations with PHH/endothelial cocultures are not entirely consistent with previously published data in cocultures of rat hepatocytes and endothelial cells, which showed relatively stable functions for several weeks. For instance, the Noh group has shown that primary rat hepatocytes displayed relatively stable urea secretion for ∼30 days when cocultivated with immortalized bovine aortic endothelial cells.17, 21 In contrast, our use of an immortalized human liver endothelial cell line (TMNK) did not lead to induction of high and stable PHH functions; we suspect that species-specific differences may be important in the discrepancies observed across the 2 studies, although we cannot entirely rule out the differences between the use of endothelial cells from different organ systems. In a more directly comparable study using primary rat hepatocyte and primary rat LSEC cocultures, Bale et al18 showed relatively stable albumin and urea secretions, and CYP1A activity over 4 weeks. Some key differences with our study include the use of high concentration (∼1 mg/mL) collagen I gels by Bale et al18 to sandwich hepatocytes and LSECs in various layers and species-specific differences in hepatocyte-LSEC interactions. Here, we did not use high concentration collagen I gels because they are (1) difficult to miniaturize into 24-well and smaller plate formats (ie, tend to be inconsistent in gelation height throughout the well and tend to peel off over a few days because of cell contraction), (2) can bind drugs when being used for screening assays, and (3) may represent a more fibrotic state of the liver in which the diverse collagens in the liver get replaced with high levels of collagen I.37, 38 Thus, because of such limitations, collagen I gel–based liver models are not routinely used in pharmaceutical practice.39, 40 Ultimately, our approach/model is human-specific and is more amenable to high-throughput applications in the drug development pipeline.
In contrast to declining functions in PHH/endothelial cocultures, all the PHH functions measured (albumin and urea secretions, and CYP2A6 and CYP3A4 enzyme activities) were significantly higher and stable over 3 weeks in cocultures of PHHs and 3T3-J2 murine embryonic fibroblasts. This mouse fibroblast cell line can induce functions in PHHs at levels closer to those observed in freshly isolated PHHs from the same donors.41 More broadly, coculture with both liver- and nonliver-derived NPC types, including those of mouse 3T3 fibroblast origin,28, 42, 43, 44, 45, 46, 47, 48 has been long known to induce functions in primary hepatocytes from multiple species, including humans, which suggests that the molecular mediators underlying the coculture effect are relatively well-conserved across species.18, 49 Although the complete mechanism underlying the effects of 3T3-J2 fibroblasts on PHHs remains to be elucidated, 3T3-J2 fibroblasts express various molecules found in the liver, such as decorin,28 vascular endothelial growth factor-D,28 and T-cadherin,50 which have been implicated in the ability of these fibroblasts to induce functions in hepatocytes from multiple species.40 Furthermore, 3T3-J2 fibroblasts have a lack of detectable liver functions, display contact inhibition that avoids overgrowth, and are propagated easily before inclusion in coculture.12, 16, 28, 51 More importantly, the use of 3T3-J2 fibroblasts in coculture with PHHs does not prevent the effective use of the stabilized PHHs for many applications in drug development, such as drug clearance prediction,36, 52 drug metabolite identification,53 drug-transporter interactions,54 drug hepatotoxicity,10 hepatitis B/C viral infections,55, 56 malaria infection,57, 58 and steatosis and insulin resistance caused by hyperglycemia as in diabetes.13, 31
We used the previously mentioned coculture effect here to create a triculture model in which 3T3-J2 fibroblasts were used to stabilize PHHs to functional levels closer to physiological outcomes than possible with endothelial cells (LSECs or HUVECs), and endothelial cells were introduced within the fibroblast monolayer at a physiologic ratio (1 endothelial cell:5 PHHs) to allow PHH/endothelial interactions as in vivo. Tricultures were created in both coplanar (ie, all 3 cell types could interact via paracrine and contact signaling) and layered (separation of PHHs and LSECs via a gelled Matrigel layer to mimic the space of Disse) configurations. Matrigel is widely used to overlay PHHs with an ECM gel because it has diverse components, many of which are present in the liver (eg, different collagens types instead of collagen I alone and laminins).11, 59, 60, 61 The projected surface area, functions (albumin and urea secretions, and CYP3A4/2A6 enzyme activities), and active bile canaliculi of PHHs in both triculture configurations were remarkably similar to those observed in PHH/fibroblast cocultures, suggesting that the ability of the fibroblasts to induce and stabilize functions in PHHs is not compromised by inclusion of endothelial cells; such an approach enables a well-differentiated PHH phenotype independently (via the 3T3-J2 fibroblast support) of the PHHs’ ability to interact with endothelial cells. Both the coplanar and layered tricultures were statistically similar with respect to PHH functions, suggesting that the in vivo–like separation between endothelial cells and PHHs does not induce greater levels of functions than when the cell types can interact via both paracrine and contact signaling. We selected the coplanar triculture configuration for all other studies because the lack of protein gels is preferred for drug development applications as described previously. Nonetheless, the layered configuration can be highly useful when subjecting the tricultures to microfluidic perfusion in liver-on-a-chip platforms because it exposes the endothelial cells to shear stress while protecting the PHHs from shear stress as in vivo.
Although we are the first group to our knowledge to create a human-relevant triculture platform using PHHs, fibroblasts, and primary human endothelial cells (LSECs and HUVECs), our findings are consistent with triculture data obtained in rat liver platforms. For instance, March et al15 combined primary rat hepatocytes and primary rat LSECs with 3T3 fibroblasts on a mechanically actuated dynamic substrate; this triculture model better preserved the phenotype of hepatocytes and fenestrations of LSECs relative to cultures without fibroblasts. In another study, Liu et al62 found that their triculture system containing primary rat hepatocytes with 3T3 fibroblasts and HUVECs functionally outperformed the hepatocyte/endothelial coculture control. These findings suggest that 3T3 fibroblasts can stabilize hepatocyte and endothelial functions across rodent and human species, which bodes well for use of hepatocyte/fibroblast/endothelial cell tricultures for elucidating specific-specific mechanisms underlying physiological and pathophysiological phenomena. However, for drug development applications, well-documented differences in drug metabolism and toxicity pathways between rodents and humans8, 9, 10 necessitate the use of human-relevant liver models; our platform now provides the avenue for predicting the effects of drugs that act on PHHs and/or primary human LSECs.
The sourcing of endothelial cells is a major consideration for building in vitro models of the human liver. Ideally, freshly isolated LSECs from human liver tissue would be used in all applications because they are the closest representation of human liver physiology; however, the routine use of this gold standard cell type is not practical for drug screening applications that necessitates creation of on-demand cultures from the same donors via the use of cryopreserved cells toward mitigating donor-to-donor variability when testing a large number of compounds longitudinally.11, 63 Because of such limitations with freshly isolated primary human LSECs, most other groups rely on either immortalized human endothelial cells23 or endothelial cells from other species and/or organ systems21 when developing culture platforms for drug development. Several studies have cocultivated hepatocytes with the TMNK immortalized human liver endothelial cells because of their ease of propagation64, 65, 66; however, here we show that in contrast to primary endothelial cells, TMNK cells cause a severe decline in PHH morphology and functions with or without fibroblasts, likely because of overgrowth. We were able to passage primary human LSECs from multiple donors at least 6 times and use them subsequently in coculture and triculture studies. Nonetheless, because it is not trivial to commercially source primary human LSECs from more than a few donors, we also evaluated the effects of HUVECs, which are readily available from many donors and have a precedence for use in cocultures with primary hepatocytes and stem cell–derived hepatocyte-like cells.22, 67, 68, 69, 70 Our results here indicate that PHH/fibroblast/endothelial cell tricultures containing HUVECs display similar levels and longevity of PHH functions over 3 weeks, and thus such tricultures can serve as a first approximation when modeling PHH/endothelial interactions, whereas primary human LSECs can be used in select studies to elucidate similarities and differences with reciprocal interactions between PHHs and nonliver-endothelial cells.
To determine endothelial phenotype in tricultures, we first evaluated gene expression of CD31, CD54, F8, and vWF, because these markers have been shown to be consistently expressed in human liver slices and isolated LSECs.71, 72, 73, 74 CD31 is a member of the immunoglobulin superfamily known to be expressed in many types of endothelial cells, but specifically in the cytoplasm of LSECs.4, 75 CD54 is a member of the immunoglobulin superfamily expressed on the surface of endothelial cells and implicated in various signaling pathways, including some immune pathways.76, 77 vWF is a multimeric glycoprotein that mediates platelet adhesion and thrombus formation during vascular injury,78 whereas F8 is a coagulation factor that is carried by vWF in circulating blood that leads to normal arrest of bleeding and thrombus formation.78, 79 All of the previously mentioned gene expression markers were detected in both PHH/endothelial cocultures and PHH/fibroblast/endothelial tricultures for 3 weeks.
The widely accepted hallmark of LSEC phenotype is the presence of fenestrations, which are responsible for the exchange of soluble and particulate material between the blood and the space of Disse.80, 81 The pattern of fenestration has been extensively studied in rat and mouse LSECs,15, 81, 82, 83, 84 but has been shown to be similar in human LSECs.85 The distribution of fenestrations is highly dynamic, both with respect to location in the liver and grouping with adjacent fenestrations.81, 86 Furthermore, the number and diameter of fenestrations have been shown to correlate strongly with liver conditions (eg, fatty liver, hepatitis, and hepatectomy) and exposure to xenobiotic and environmental agents (eg, ethanol, nicotine).81 It has also been suggested that the diameter of fenestrations affects the uptake and transport of lipoproteins in the sinusoid, which may greatly impact the pathogenesis of atherosclerosis.81 In this study, we detected the presence of fenestrations on LSECs via SEM in pure cultures, PHH/LSECs cell cocultures, and PHH/fibroblast/LSEC tricultures; however, we were not able to elucidate quantitative differences across the culture configurations using SEM analysis. Nonetheless, coupled with gene expression analysis, the presence of fenestrations suggests that both cocultures and tricultures can maintain the survival of endothelial cells irrespective of functional levels in PHHs. However, in contrast to PHH/endothelial cocultures that display declining PHH functions, tricultures are better suited to evaluate reciprocal interactions between stable endothelial cells and PHHs in physiologic and pathophysiologic conditions. For instance, our tricultures could be used to model in vivo–like paracrine signaling between hepatitis C virus–infected PHHs and LSECs, because infected LSECs are known to release exosomes and inhibit viral replication in infected hepatocytes, albeit from a cancerous origin.5 Furthermore, some drugs are known to cause toxicity to LSECs,7, 87 which can lead to downstream effects in PHHs because of the release of apoptotic factors from the LSECs; our tricultures can serve to elucidate such crosstalk following drug exposure.
Our goal in this study was to determine how primary human endothelial cells (LSECs and HUVECs) affect long-term PHH functions relative to 3T3-J2 murine embryonic fibroblasts and then construct a triculture platform that can enable stable phenotypes of both PHHs and endothelial cells for several weeks. However, other NPC types in the liver, such as hepatic stellate cells and Kupffer cells/macrophages, also interact with PHHs and LSECs in vivo. The use of the 3T3-J2 fibroblasts to stabilize PHH functions allows us to introduce specific liver NPC types within and around the fibroblast monolayer to study interactions with PHHs, whereas the use of PHH micropatterning allows us to control for PHH homotypic contacts that are critical for establishment of hepatocyte polarity, such as the formation of cell junctions (eg, cadherins) and bile canaliculi. Indeed, Nguyen et al88 showed that primary human Kupffer cells can be cultured atop pre-established micropatterned cocultures containing PHHs and fibroblasts to study the effects of Kupffer cell activation on hepatic CYP450s. Similarly, Davidson et al89 showed that primary human activated (fibrogenic) hepatic stellate cells can be cultured within the fibroblast monolayer surrounding the PHH micropatterned colonies to model an early nonalcoholic steatohepatitis-like phenotype in the PHHs, which could be alleviated with clinically relevant drugs. Thus, PHH/fibroblast/liver NPC triculture configurations offer robust in vitro tools to elucidate reciprocal interactions between PHHs and liver NPCs in physiological/disease contexts and for drug screening.
In conclusion, we showed here that neither primary human LSECs nor nonliver HUVECs can stabilize the PHH phenotype over several weeks, which necessitated the use of 3T3-J2 fibroblasts in a PHH/fibroblast/endothelial cell triculture configuration that subsequently led to high levels of prototypical hepatic functions and endothelial phenotype for at least 3 weeks in vitro. Separating the endothelial cells from the PHHs via a thin protein gel (Matrigel) to mimic the space of Disse as in vivo did not lead to better PHH functions than the coplanar tricultures, although both configurations can have utility for addressing specific liver-specific hypotheses as detailed previously. Ultimately, coupling PHH/fibroblast/liver NPC tricultures with cultures created from other tissues on a microfluidic chip (ie, body-on-a-chip) will allow a systems-level exploration of disease progression and the effects of drugs on multiple interacting organ systems.
Acknowledgments
The authors thank Dr. Hugo Rosen (University of Colorado-Denver Medical School) for providing primary liver sinusoidal endothelial cells and TMNK cells and Samuel Allsup, Matthew Davidson, Christine Lin, and Wendy Sunada for assistance with cell culture. This work made use of instruments in the Electron Microscopy Service (Research Resources Center, University of Illinois at Chicago).
Footnotes
Author contributions All authors conceived experiments. Brenton R. Ware, Mitchell J. Durham, and Chase P. Monckton executed the experiments. Brenton R. Ware and Salman R. Khetani wrote the manuscript.
Conflicts of interest This author discloses the following: Salman R. Khetani is an equity holder in Ascendance Biotechnology, which has licensed the micropatterned cocultures and related systems from MIT and Colorado State University for commercial distribution. The remaining authors disclose no conflicts.
Funding This work was funded by the National Institutes of Health (1R21ES027622-01, S.R.K.) and National Science Foundation (CBET-1351909, S.R.K.).
References
- 1.Smedsrød B., Pertoft H., Gustafson S., Laurent T.C. Scavenger functions of the liver endothelial cell. Biochem J. 1990;266:313–327. doi: 10.1042/bj2660313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Limmer A., Ohl J., Kurts C., Ljunggren H.G., Reiss Y., Groettrup M., Momburg F., Arnold B., Knolle P.A. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat Med. 2000;6:1348–1354. doi: 10.1038/82161. [DOI] [PubMed] [Google Scholar]
- 3.Do H., Healey J.F., Waller E.K., Lollar P. Expression of factor VIII by murine liver sinusoidal endothelial cells. Journal of Biological Chemistry. 1999;274:19587–19592. doi: 10.1074/jbc.274.28.19587. [DOI] [PubMed] [Google Scholar]
- 4.DeLeve L.D. Liver sinusoidal endothelial cells and liver regeneration. J Clin Invest. 2013;123:1861–1866. doi: 10.1172/JCI66025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giugliano S., Kriss M., Golden-Mason L., Dobrinskikh E., Stone A.E.L., Soto-Gutierrez A., Mitchell A., Khetani S.R., Yamane D., Stoddard M., Li H., Shaw G.M., Edwards M.G., Lemon S.M., Gale M., Shah V.H., Rosen H.R. Hepatitis C virus infection induces autocrine interferon signaling by human liver endothelial cells and release of exosomes, which inhibits viral replication. Gastroenterology. 2015;148:392–402. doi: 10.1053/j.gastro.2014.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maslak E., Gregorius A., Chlopicki S. Liver sinusoidal endothelial cells (LSECs) function and NAFLD; NO-based therapy targeted to the liver. Pharmacol Rep. 2015;67:689–694. doi: 10.1016/j.pharep.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 7.DeLeve L.D., Wang X., Kuhlenkamp J.F., Kaplowitz N. Toxicity of azathioprine and monocrotaline in murine sinusoidal endothelial cells and hepatocytes: the role of glutathione and relevance to hepatic venoocclusive disease. Hepatology. 1996;23:589–599. doi: 10.1002/hep.510230326. [DOI] [PubMed] [Google Scholar]
- 8.Olson H., Betton G., Robinson D., Thomas K., Monro A., Kolaja G., Lilly P., Sanders J., Sipes G., Bracken W., Dorato M., Van Deun K., Smith P., Berger B., Heller A. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul Toxicol Pharmacol. 2000;32:56–67. doi: 10.1006/rtph.2000.1399. [DOI] [PubMed] [Google Scholar]
- 9.Shih H., Pickwell G.V., Guenette D.K., Bilir B., Quattrochi L.C. Species differences in hepatocyte induction of CYP1A1 and CYP1A2 by omeprazole. Hum Exp Toxicol. 1999;18:95–105. doi: 10.1177/096032719901800206. [DOI] [PubMed] [Google Scholar]
- 10.Khetani S.R., Kanchagar C., Ukairo O., Krzyzewski S., Moore A., Shi J., Aoyama S., Aleo M., Will Y. Use of micropatterned cocultures to detect compounds that cause drug-induced liver injury in humans. Toxicol Sci. 2013;132:107–117. doi: 10.1093/toxsci/kfs326. [DOI] [PubMed] [Google Scholar]
- 11.Godoy P., Hewitt N.J., Albrecht U., Andersen M.E., Ansari N., Bhattacharya S., Bode J.G., Bolleyn J., Borner C., Böttger J., Braeuning A., Budinsky R.A., Burkhardt B., Cameron N.R., Camussi G., Cho C.-S., Choi Y.-J., Craig Rowlands J., Dahmen U., Damm G., Dirsch O., Donato M.T., Dong J., Dooley S., Drasdo D., Eakins R., Ferreira K.S., Fonsato V., Fraczek J., Gebhardt R., Gibson A., Glanemann M., Goldring C.E.P., Gómez-Lechón M.J., Groothuis G.M.M., Gustavsson L., Guyot C., Hallifax D., Hammad S., Hayward A., Häussinger D., Hellerbrand C., Hewitt P., Hoehme S., Holzhütter H.-G., Houston J.B., Hrach J., Ito K., Jaeschke H., Keitel V., Kelm J.M., Kevin Park B., Kordes C., Kullak-Ublick G.A., LeCluyse E.L., Lu P., Luebke-Wheeler J., Lutz A., Maltman D.J., Matz-Soja M., McMullen P., Merfort I., Messner S., Meyer C., Mwinyi J., Naisbitt D.J., Nussler A.K., Olinga P., Pampaloni F., Pi J., Pluta L., Przyborski S.A., Ramachandran A., Rogiers V., Rowe C., Schelcher C., Schmich K., Schwarz M., Singh B., Stelzer E.H.K., Stieger B., Stöber R., Sugiyama Y., Tetta C., Thasler W.E., Vanhaecke T., Vinken M., Weiss T.S., Widera A., Woods C.G., Xu J.J., Yarborough K.M., Hengstler J.G. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch Toxicol. 2013;87:1315–1530. doi: 10.1007/s00204-013-1078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khetani S.R., Bhatia S.N. Microscale culture of human liver cells for drug development. Nat Biotechnol. 2008;26:120–126. doi: 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- 13.Davidson M.D., Lehrer M., Khetani S.R. Hormone and drug-mediated modulation of glucose metabolism in a microscale model of the human liver. Tissue Eng Part C Methods. 2015;21:716–725. doi: 10.1089/ten.tec.2014.0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Godoy P., Widera A., Schmidt-Heck W., Campos G., Meyer C., Cadenas C., Reif R., Stöber R., Hammad S., Pütter L., Gianmoena K., Marchan R., Ghallab A., Edlund K., Nussler A., Thasler W.E., Damm G., Seehofer D., Weiss T.S., Dirsch O., Dahmen U., Gebhardt R., Chaudhari U., Meganathan K., Sachinidis A., Kelm J., Hofmann U., Zahedi R.P., Guthke R., Blüthgen N., Dooley S., Hengstler J.G. Gene network activity in cultivated primary hepatocytes is highly similar to diseased mammalian liver tissue. Arch Toxicol. 2016;90:2513–2529. doi: 10.1007/s00204-016-1761-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.March S., Hui E.E., Underhill G.H., Khetani S.R., Bhatia S.N. Microenvironmental regulation of the sinusoidal endothelial cell phenotype in vitro. Hepatology. 2009;50:920–928. doi: 10.1002/hep.23085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhatia S.N., Balis U.J., Yarmush M.L., Toner M. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13:1883–1900. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- 17.Kang Y.B.A., Rawat S., Cirillo J., Bouchard M., Noh H.M. Layered long-term co-culture of hepatocytes and endothelial cells on a transwell membrane: toward engineering the liver sinusoid. Biofabrication. 2013;5:045008. doi: 10.1088/1758-5082/5/4/045008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bale S.S., Golberg I., Jindal R., McCarty W.J., Luitje M., Hegde M., Bhushan A., Usta O.B., Yarmush M.L. Long-term coculture strategies for primary hepatocytes and liver sinusoidal endothelial cells. Tissue Eng Part C Methods. 2015;21:413–422. doi: 10.1089/ten.tec.2014.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim K., Ohashi K., Utoh R., Kano K., Okano T. Preserved liver-specific functions of hepatocytes in 3D co-culture with endothelial cell sheets. Biomaterials. 2012;33:1406–1413. doi: 10.1016/j.biomaterials.2011.10.084. [DOI] [PubMed] [Google Scholar]
- 20.Kim Y., Rajagopalan P. 3D hepatic cultures simultaneously maintain primary hepatocyte and liver sinusoidal endothelial cell phenotypes. PLoS One. 2010;5:e15456. doi: 10.1371/journal.pone.0015456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang Y.B.A., Sodunke T.R., Lamontagne J., Cirillo J., Rajiv C., Bouchard M.J., Noh M. Liver sinusoid on a chip: long-term layered co-culture of primary rat hepatocytes and endothelial cells in microfluidic platforms. Biotechnol Bioeng. 2015;112:2571–2582. doi: 10.1002/bit.25659. [DOI] [PubMed] [Google Scholar]
- 22.Ho C.T., Lin R.Z., Chen R.J., Chin C.K., Gong S.E., Chang H.Y., Peng H.L., Hsu L., Yew T.R., Chang S.F., Liu C.H. Liver-cell patterning lab chip: mimicking the morphology of liver lobule tissue. Lab Chip. 2013;13:3578–3587. doi: 10.1039/c3lc50402f. [DOI] [PubMed] [Google Scholar]
- 23.Vernetti L.A., Senutovitch N., Boltz R., Debiasio R., Shun T.Y., Gough A., Taylor D.L. A human liver microphysiology platform for investigating physiology, drug safety, and disease models. Exp Biol Med. 2016;241:101–114. doi: 10.1177/1535370215592121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohno M., Motojima K., Okano T., Taniguchi A. Induction of drug-metabolizing enzymes by phenobarbital in layered co-culture of a human liver cell line and endothelial cells. Biol Pharm Bull. 2009;32:813–817. doi: 10.1248/bpb.32.813. [DOI] [PubMed] [Google Scholar]
- 25.Jindal R., Patel S.J., Yarmush M.L. Tissue-engineered model for real-time monitoring of liver inflammation. Tissue Eng Part C Methods. 2011;17:113–122. doi: 10.1089/ten.tec.2009.0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nahmias Y., Casali M., Barbe L., Berthiaume F., Yarmush M.L. Liver endothelial cells promote LDL-R expression and the uptake of HCV-like particles in primary rat and human hepatocytes. Hepatology. 2006;43:257–265. doi: 10.1002/hep.21016. [DOI] [PubMed] [Google Scholar]
- 27.Kidambi S., Yarmush R.S., Novik E., Chao P., Yarmush M.L., Nahmias Y. Oxygen-mediated enhancement of primary hepatocyte metabolism, functional polarization, gene expression, and drug clearance. Proc Natl Acad Sci. 2009;106:15714–15719. doi: 10.1073/pnas.0906820106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khetani S.R., Szulgit G., Del Rio J.A., Barlow C., Bhatia S.N. Exploring interactions between rat hepatocytes and nonparenchymal cells using gene expression profiling. Hepatology. 2004;40:545–554. doi: 10.1002/hep.20351. [DOI] [PubMed] [Google Scholar]
- 29.March S., Ramanan V., Trehan K., Ng S., Galstian A., Gural N., Scull M.A., Shlomai A., Mota M.M., Fleming H.E., Khetani S.R., Rice C.M., Bhatia S.N. Micropatterned coculture of primary human hepatocytes and supportive cells for the study of hepatotropic pathogens. Nat Protoc. 2015;10:2027–2053. doi: 10.1038/nprot.2015.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsumura T., Takesue M., Westerman K.A., Okitsu T., Sakaguchi M., Fukazawa T., Totsugawa T., Noguchi H., Yamamoto S., Stolz D.B., Tanaka N., Leboulch P., Kobayashi N. Establishment of an immortalized human-liver endothelial cell line with SV40T and hTERT. Transplantation. 2004;77:1357–1365. doi: 10.1097/01.tp.0000124286.82961.7e. [DOI] [PubMed] [Google Scholar]
- 31.Davidson M.D., Ballinger K.R., Khetani S.R. Long-term exposure to abnormal glucose levels alters drug metabolism pathways and insulin sensitivity in primary human hepatocytes. Sci Rep. 2016;6:28178. doi: 10.1038/srep28178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LeCluyse E.L., Witek R.P., Andersen M.E., Powers M.J. Organotypic liver culture models: meeting current challenges in toxicity testing. Crit Rev Toxicol. 2012;42:501–548. doi: 10.3109/10408444.2012.682115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collins T.J. ImageJ for microscopy. BioTechniques. 2007;43:25–30. doi: 10.2144/000112517. [DOI] [PubMed] [Google Scholar]
- 34.Chen A.A., Thomas D.K., Ong L.L., Schwartz R.E., Golub T.R., Bhatia S.N. Humanized mice with ectopic artificial liver tissues. Proc Natl Acad Sci. 2011;108:11842–11847. doi: 10.1073/pnas.1101791108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zamek-Gliszczynski M.J., Xiong H., Patel N.J., Turncliff R.Z., Pollack G.M., Brouwer K.L.R. Pharmacokinetics of 5 (and 6)-carboxy-2′,7′-dichlorofluorescein and its diacetate promoiety in the liver. J Pharmacol Exp Ther. 2003;304:801–809. doi: 10.1124/jpet.102.044107. [DOI] [PubMed] [Google Scholar]
- 36.Lin C., Shi J., Moore A., Khetani S.R. Prediction of drug clearance and drug-drug interactions in microscale cultures of human hepatocytes. Drug Metab Dis. 2016;44:127–136. doi: 10.1124/dmd.115.066027. [DOI] [PubMed] [Google Scholar]
- 37.Norona L.M., Nguyen D.G., Gerber D.A., Presnell S.C., LeCluyse E.L. Modeling compound-induced fibrogenesis in vitro using three-dimensional bioprinted human liver tissues. Toxicol Sci. 2016;154:354–367. doi: 10.1093/toxsci/kfw169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perepelyuk M., Terajima M., Wang A.Y., Georges P.C., Janmey P.A., Yamauchi M., Wells R.G. Hepatic stellate cells and portal fibroblasts are the major cellular sources of collagens and lysyl oxidases in normal liver and early after injury. Am J Physiol Gastrointest Liver Physiol. 2013;304:G605–G614. doi: 10.1152/ajpgi.00222.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Proctor W.R., Foster A.J., Vogt J., Summers C., Middleton B., Pilling M.A., Shienson D., Kijanska M., Strobel S., Kelm J.M., Morgan P., Messner S., Williams D. Utility of spherical human liver microtissues for prediction of clinical drug-induced liver injury. Arch Toxicol. 2017;91:2849–2863. doi: 10.1007/s00204-017-2002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khetani S.R., Berger D.R., Ballinger K.R., Davidson M.D., Lin C., Ware B.R. Microengineered liver tissues for drug testing. J Lab Autom. 2015;20:216–250. [Google Scholar]
- 41.Berger D.R., Ware B.R., Davidson M.D., Allsup S.R., Khetani S.R. Enhancing the functional maturity of induced pluripotent stem cell-derived human hepatocytes by controlled presentation of cell-cell interactions in vitro. Hepatology. 2015;61:1370–1381. doi: 10.1002/hep.27621. [DOI] [PubMed] [Google Scholar]
- 42.Cho C.H., Park J., Nagrath D., Tilles A.W., Berthiaume F., Toner M., Yarmush M.L. Oxygen uptake rates and liver-specific functions of hepatocyte and 3T3 fibroblast co-cultures. Biotechnol Bioeng. 2007;97:188–199. doi: 10.1002/bit.21225. [DOI] [PubMed] [Google Scholar]
- 43.Chia S.-M., Lin P.-C., Yu H. TGF-beta1 regulation in hepatocyte-NIH3T3 co-culture is important for the enhanced hepatocyte function in 3D microenvironment. Biotechnol Bioeng. 2005;89:565–573. doi: 10.1002/bit.20372. [DOI] [PubMed] [Google Scholar]
- 44.De La Vega F.M., Mendoza-Figueroa T. Dimethyl sulfoxide enhances lipid synthesis and secretion by long-term cultures of adult rat hepatocytes. Biochimie. 1991;73:621–624. doi: 10.1016/0300-9084(91)90033-w. [DOI] [PubMed] [Google Scholar]
- 45.Donato M.T., Gomez-Lechon M.J., Castell J.V. Drug metabolizing enzymes in rat hepatocytes co-cultured with cell lines. Vitro Cell Dev Biol. 1990;26:1057–1062. doi: 10.1007/BF02624440. [DOI] [PubMed] [Google Scholar]
- 46.Kuri-Harcuch W., Mendoza-Figueroa T. Cultivation of adult rat hepatocytes on 3T3 cells: expression of various liver differentiated functions. Differentiation. 1989;41:148–157. doi: 10.1111/j.1432-0436.1989.tb00742.x. [DOI] [PubMed] [Google Scholar]
- 47.Goulet F., Normand C., Morin O. Cellular interactions promote tissue-specific function, biomatrix deposition, and junctional communication of primary cultured hepatocytes. Hepatology. 1988;8:1010–1018. doi: 10.1002/hep.1840080506. [DOI] [PubMed] [Google Scholar]
- 48.Shimaoka S., Nakamura T., Ichihara A. Stimulation of growth of primary cultured adult rat hepatocytes without growth factors by coculture with nonparenchymal liver cells. Exp Cell Res. 1987;172:228–242. doi: 10.1016/0014-4827(87)90109-1. [DOI] [PubMed] [Google Scholar]
- 49.Guillouzo A. Liver cell models in in vitro toxicology. Environ Health Perspect. 1998;106:511–532. doi: 10.1289/ehp.98106511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khetani S.R., Chen A.A., Ranscht B., Bhatia S.N. T-cadherin modulates hepatocyte functions in vitro. FASEB J. 2008;22:3768–3775. doi: 10.1096/fj.07-105155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ukairo O., Kanchagar C., Moore A., Shi J., Gaffney J., Aoyama S., Rose K.A., Krzyzewski S., McGeehan J., Andersen M.E., Khetani S.R., LeCluyse E.L. Long-term stability of primary rat hepatocytes in micropatterned cocultures. J Biochem Mol Toxicol. 2013;27:204–212. doi: 10.1002/jbt.21469. [DOI] [PubMed] [Google Scholar]
- 52.Chan T.S., Yu H., Moore A., Khetani S.R., Tweedie D. Meeting the challenge of predicting hepatic clearance of compounds slowly metabolized by cytochrome P450 using a novel hepatocyte model, HepatoPac. Drug Metab Dispos. 2013;41:2024–2032. doi: 10.1124/dmd.113.053397. [DOI] [PubMed] [Google Scholar]
- 53.Wang W.W., Khetani S.R., Krzyzewski S., Duignan D.B., Obach R.S. Assessment of a micropatterned hepatocyte coculture system to generate major human excretory and circulating drug metabolites. Drug Metab Dispos. 2010;38:1900–1905. doi: 10.1124/dmd.110.034876. [DOI] [PubMed] [Google Scholar]
- 54.Ramsden D., Tweedie D.J., Chan T.S., Taub M.E., Li Y. Bridging in vitro and in vivo metabolism and transport of faldaprevir in human using a novel cocultured human hepatocyte system, HepatoPac. Drug Metab Dispos. 2014;42:394–406. doi: 10.1124/dmd.113.055897. [DOI] [PubMed] [Google Scholar]
- 55.Ploss A., Khetani S.R., Jones C.T., Syder A.J., Trehan K., Gaysinskaya V.A., Mu K., Ritola K., Rice C.M., Bhatia S.N. Persistent hepatitis C virus infection in microscale primary human hepatocyte cultures. Proc Natl Acad Sci. 2010;107:3141–3145. doi: 10.1073/pnas.0915130107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shlomai A., Schwartz R.E., Ramanan V., Bhatta A., de Jong Y.P., Bhatia S.N., Rice C.M. Modeling host interactions with hepatitis B virus using primary and induced pluripotent stem cell-derived hepatocellular systems. Proc Natl Acad Sci. 2014;111:12193–12198. doi: 10.1073/pnas.1412631111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ng S., March S., Galstian A., Hanson K., Carvalho T., Mota M.M., Bhatia S.N. Hypoxia promotes liver stage malaria infection in primary human hepatocytes in vitro. Dis Model Mech. 2013;7:215–224. doi: 10.1242/dmm.013490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.March S., Ng S., Velmurugan S., Galstian A., Shan J., Logan D.J., Carpenter A.E., Thomas D., Sim B.K.L., Mota M.M., Hoffman S.L., Bhatia S.N. A microscale human liver platform that supports the hepatic stages of Plasmodium falciparum and vivax. Cell Host Microbe. 2013;14:104–115. doi: 10.1016/j.chom.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schug M., Heise T., Bauer A., Storm D., Blaszkewicz M., Bedawy E., Brulport M., Geppert B., Hermes M., Föllmann W., Rapp K., Maccoux L., Schormann W., Appel K.E., Oberemm A., Gundert-Remy U., Hengstler J.G. Primary rat hepatocytes as in vitro system for gene expression studies: comparison of sandwich, Matrigel, and 2D cultures. Arch Toxicol. 2008;82:923–931. doi: 10.1007/s00204-008-0375-x. [DOI] [PubMed] [Google Scholar]
- 60.Bi Y.A., Kazolias D., Duignan D.B. Use of cryopreserved human hepatocytes in sandwich culture to measure hepatobiliary transport. Drug Metab Dispos. 2006;34:1658–1665. doi: 10.1124/dmd.105.009118. [DOI] [PubMed] [Google Scholar]
- 61.Govindarajan R., Endres C.J., Whittington D., LeCluyse E., Pastor-Anglada M., Tse C.M., Unadkat J.D. Expression and hepatobiliary transport characteristics of the concentrative and equilibrative nucleoside transporters in sandwich-cultured human hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2008;295:G570–G580. doi: 10.1152/ajpgi.00542.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Y., Li H., Yan S., Wei J., Li X. Hepatocyte cocultures with endothelial cells and fibroblasts on micropatterned fibrous mats to promote liver-specific functions and capillary formation capabilities. Biomacromolecules. 2014;15:1044–1054. doi: 10.1021/bm401926k. [DOI] [PubMed] [Google Scholar]
- 63.Li A.P., Lu C., Brent J.A., Pham C., Fackett A., Ruegg C.E., Silber P.M. Cryopreserved human hepatocytes: characterization of drug-metabolizing enzyme activities and applications in higher throughput screening assays for hepatotoxicity, metabolic stability, and drug-drug interaction potential. Chem Biol Interact. 1999;121:17–35. doi: 10.1016/s0009-2797(99)00088-5. [DOI] [PubMed] [Google Scholar]
- 64.Xiao W., Perry G., Sakai Y. New physiologically-relevant liver tissue model based on hierarchically cocultured primary rat hepatocytes with liver endothelial cells. Integrative Biology. 2015;7:1412–1422. doi: 10.1039/c5ib00170f. [DOI] [PubMed] [Google Scholar]
- 65.Kawasaki T., Murata S., Takahashi K., Nozaki R., Ohshiro Y., Ikeda N., Pak S., Myronovych A., Hisakura K., Fukunaga K., Oda T., Sasaki R., Ohkohchi N. Activation of human liver sinusoidal endothelial cell by human platelets induces hepatocyte proliferation. J Hepatol. 2010;53:648–654. doi: 10.1016/j.jhep.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 66.Taylor D.P., Clark A.M., Wheeler S.E., Wells A. Hepatic nonparenchymal cells drive metastatic breast cancer outgrowth and partial epithelial to mesenchymal transition. Breast Cancer Res Treat. 2014;144:551–560. doi: 10.1007/s10549-014-2875-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma X., Qu X., Zhu W., Li Y.-S., Yuan S., Zhang H., Liu J., Wang P., Lai C.S.E., Zanella F., Feng G.-S., Sheikh F., Chien S., Chen S. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc Natl Acad Sci. 2016;113:2206–2211. doi: 10.1073/pnas.1524510113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nelson L.J., Navarro M., Treskes P., Samuel K., Tura-Ceide O., Morley S.D., Hayes P.C., Plevris J.N. Acetaminophen cytotoxicity is ameliorated in a human liver organotypic co-culture model. Sci Rep. 2015;5:17455. doi: 10.1038/srep17455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takayama G., Taniguchi A., Okano T. Identification of differentially expressed genes in hepatocyte/endothelial cell co-culture system. Tissue Eng. 2007;13:159–166. doi: 10.1089/ten.2006.0143. [DOI] [PubMed] [Google Scholar]
- 70.Guzzardi M.A., Vozzi F., Ahluwalia A.D. Study of the crosstalk between hepatocytes and endothelial cells using a novel multicompartmental bioreactor: a comparison between connected cultures and cocultures. Tissue Eng Part A. 2009;15:3635–3644. doi: 10.1089/ten.TEA.2008.0695. [DOI] [PubMed] [Google Scholar]
- 71.Scoazec J.-Y., Feldmann G. The cell adhesion molecules of hepatic sinusoidal endothelial cells. J Hepatol. 1994;20:296–300. doi: 10.1016/s0168-8278(05)80072-8. [DOI] [PubMed] [Google Scholar]
- 72.Scoazec J.-Y., Feldmann G. In situ immunophenotyping study of endothelial cells of the human hepatic sinusoid: results and functional implications. Hepatology. 1991;14:789–797. doi: 10.1002/hep.1840140508. [DOI] [PubMed] [Google Scholar]
- 73.Karrar A., Broomé U., Uzunel M., Qureshi A.R., Sumitran-Holgersson S. Human liver sinusoidal endothelial cells induce apoptosis in activated T cells: a role in tolerance induction. Gut. 2007;56:243–252. doi: 10.1136/gut.2006.093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van der Kwast T.H., Stel H.V., Cristen E., Bertina R.M., Veerman E.C.I. Localization of factor VIII-procoagulant antigen: an immunohistological survey of the human body using monoclonal antibodies. Blood. 1986;67:222–227. [PubMed] [Google Scholar]
- 75.Neubauer K., Wilfling T., Ritzel A., Ramadori G. Platelet-endothelial cell adhesion molecule-1 gene expression in liver sinusoidal endothelial cells during liver injury and repair. J Hepatol. 2000;32:921–932. doi: 10.1016/s0168-8278(00)80096-3. [DOI] [PubMed] [Google Scholar]
- 76.Wolf S.I., Howat S., Abraham D.J., Pearson J.D., Lawson C. Agonistic anti-ICAM-1 antibodies in scleroderma: activation of endothelial pro-inflammatory cascades. Vasc Pharmacol. 2013;59:19–26. doi: 10.1016/j.vph.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhu X.-W., Gong J.-P. Expression and role of ICAM-1 in the occurrence and development of hepatocellular carcinoma. Asian Pacific Journal of Cancer Prevention. 2013;14:1579–1583. doi: 10.7314/apjcp.2013.14.3.1579. [DOI] [PubMed] [Google Scholar]
- 78.Ruggeri Z.M., Ware J. Von Willebrand factor. FASEB J. 1993;7:308–316. doi: 10.1096/fasebj.7.2.8440408. [DOI] [PubMed] [Google Scholar]
- 79.Shahani T., Covens K., Lavend'homme R., Jazouli N., Sokal E.M., Peerlinck K., Jacquemin M. Human liver sinusoidal endothelial cells but not hepatocytes contain factor VIII. J Thromb Haemost. 2014;12:36–42. doi: 10.1111/jth.12412. [DOI] [PubMed] [Google Scholar]
- 80.Wisse E., De Zanger R.B., Chareis K., Van Der Smizzen P., McCuskey R.S. The liver sieve: considerations concerning the structure and function of endothelial fenestrae, the sinusoidal wall, and the space of Disse. Hepatology. 1985;5:683–692. doi: 10.1002/hep.1840050427. [DOI] [PubMed] [Google Scholar]
- 81.Braet F., Wisse E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comp Hepatol. 2002;1:1–17. doi: 10.1186/1476-5926-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.DeLeve L.D., Wang X., Guo Y. Sinusoidal endothelial cells prevent rat stellate cell activation and promote reversion to quiescence. Hepatology. 2008;48:920–930. doi: 10.1002/hep.22351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fraser R., Clark S.A., Day W.A., Murray F.E. Nicotine decreases the porosity of the rat liver sieve: a possible mechanism for hypercholesterolaemia. Br J Exp Pathol. 1988;69:345–350. [PMC free article] [PubMed] [Google Scholar]
- 84.Carpenter B., Lin Y., Stoll S., Raffai R.L., McCuskey R.S., Wang R. VEGF is crucial for the hepatic vascular development required for lipoprotein uptake. Development. 2005;132:3293–3303. doi: 10.1242/dev.01902. [DOI] [PubMed] [Google Scholar]
- 85.Horn T., Christoffersen P., Henriksen J.H. Alcoholic liver injury: defenestration in noncirrhotic livers. A scanning electron microscopic study. Hepatology. 1987;7:77–82. doi: 10.1002/hep.1840070117. [DOI] [PubMed] [Google Scholar]
- 86.Yokomori H., Oda M., Yoshimura K., Hibi T. Recent advances in liver sinusoidal endothelial ultrastructure and fine structure immunocytochemistry. Micron. 2012;43:129–134. doi: 10.1016/j.micron.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 87.DeLeve L.D. Dacarbazine toxicity in murine liver cells: a model of hepatic endothelial injury and glutathione defense. J Pharmacol Exp Ther. 1994;268:1261–1270. [PubMed] [Google Scholar]
- 88.Nguyen T.V., Ukairo O., Khetani S.R., McVay M., Kanchagar C., Seghezzi W., Ayanoglu G., Irrechukwu O., Evers R. Establishment of a hepatocyte-Kupffer cell coculture model for assessment of proinflammatory cytokine effects on metabolizing enzymes and drug transporters. Drug Metab Dispos. 2015;43:774–785. doi: 10.1124/dmd.114.061317. [DOI] [PubMed] [Google Scholar]
- 89.Davidson M.D., Kukla D.A., Khetani S.R. Microengineered cultures containing human hepatic stellate cells and hepatocytes for drug development. Integrative Biology. 2017;9:662–677. doi: 10.1039/c7ib00027h. [DOI] [PubMed] [Google Scholar]