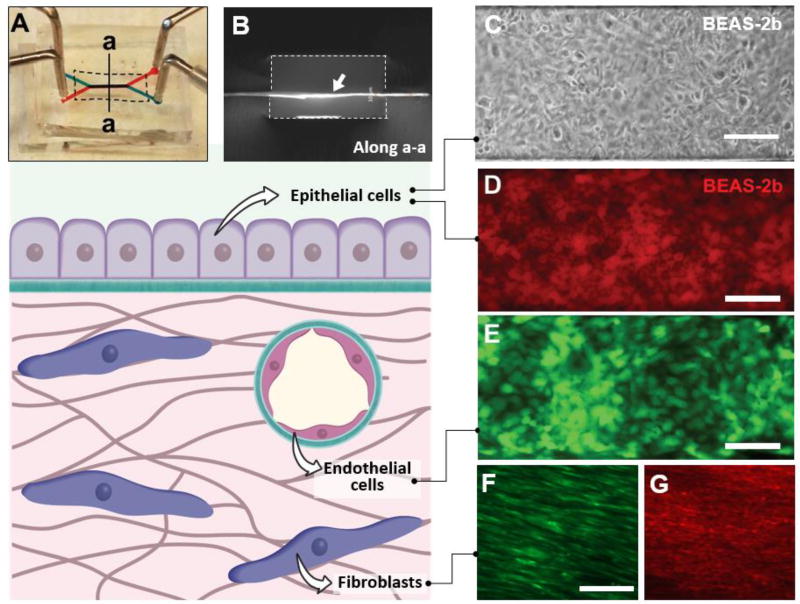
Figure 4. Microfluidic cell culture using ECM-derived membranes.
A. Digital photograph of a microfluidic cell culture device comprised of upper (blue) and lower (red) microchannels separated by a COL-MAT membrane (shown with dotted line). Injection of red and blue food coloring dyes demonstrates patency of device bonding and partitioning function of the membrane. B. A cross-sectional view of the device along the line a-a shown in A. The ECM membrane indicated with a white arrow was immunostained to visualize type I collagen. The dotted lines show the channel walls. Channel width = 500 microns. C. Phase contrast micrograph of a confluent human bronchial epithelial cell (BEAS-2b) monolayer formed on a COL-membrane in a three-layer microdevice. The cells were cultured for 72 hours under flow conditions at a flow rate of 100 µl/hr. Scale bar = 200 µm. D. A confluent monolayer of BEAS-2b cells stained with red CellTracker dye. Scale bar = 200 µm. E. Human umbilical vein endothelial cells (HUVEC) grown to confluence on the surface of COL-MAT membrane visualized by fluorescence imaging of constitutive green fluorescent protein (GFP, green) expression following 48 hours of microfluidic perfusion culture at 100 µl/hour. Scale bar = 200 µm. F. Normal human lung fibroblasts (NHLFs) growing on the COL-MAT membrane surface. Red CellTracker dye was used to visualize the cells. Scale bar = 100 µm. G. Immunofluorescence staining of alpha smooth muscle actin (α-SMA) in NHLFs cultured in the microdevice. Scale bar = 100 µm.