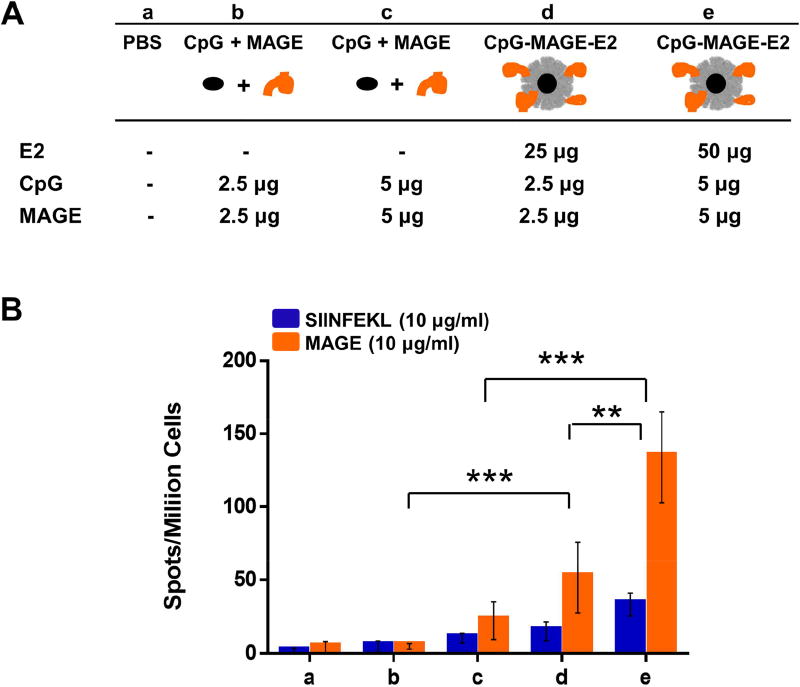
Figure 4. ELISpot analysis of splenocytes from immunization with MAGE-A3 formulations.
A) Vaccine components per dose of different formulation groups (a–e). B) Summary of averaged ELISpot data, which evaluated antigen-specific IFN-γ secretion. HLA-A2 mice were immunized with different formulations (a–e), and splenocytes were pulsed ex vivo in the presence of relevant peptide (MAGE) or irrelevant peptide (SIINFEKL) and analyzed for specific IFN-γ secretion. Higher MAGE-A3 epitope-specific IFN-γ secretion was observed for the group that received CpG-MAGE-E2. Data is presented as average ± S.E.M. of 3 independent experiments (n ≥ 3). Statistical significance was determined by two-way ANOVA followed by a Tukey's test (**p < 0.01; ***p < 0.001).