Abstract
Objective
To study the combined effect of simulated occlusal loading and plaque-derived biofilm on the interfacial integrity of dental composite restorations, and to explore whether the effects are modulated by the incorporation of sucrose.
Methods
MOD-class-II restorations were prepared in third molars. Half of the specimens (n=27) were subjected to 200,000 cycles of mechanical loading using an artificial oral environment (ART). Then, both groups of specimens (fatigued and non-fatigued) were divided into three subgroups for testing in CDC-reactors under the following conditions: no biofilm (Control), biofilm with no sucrose (BNS) and biofilm pulsed with sucrose (BWS). BNS and BWS reactors were incubated with a multispecies inoculum from a single plaque donor whereas the control reactor was not. The BWS reactor was pulsed with sucrose five times a day. The biofilm challenges were repeated sequentially for 12 weeks. pH was recorded for each run. Specimens were examined for demineralization with micro-CT and load capacity by fast fracture test.
Results
Demineralization next to the restorations was only detectable in BWS teeth. Fracture loads were significantly reduced by the concomitant presence of biofilm and sucrose, regardless of whether cyclic mechanical loading was applied. Cyclic loading reduced fracture loads under all reactor conditions, but the reduction was not statistically significant.
Conclusions
Sucrose pulsing was required to induce biofilm-mediated degradation of the adhesive interface.
We have presented a comprehensive and clinically relevant model to study the effects of mechanical loading and microbial challenge on the interfacial integrity of dental restorations.
Keywords: Adhesive interface, Mechanical Fatigue, Resin Composite, Oral Biofilms, cyclic loading, sucrose
GRAPHICAL ABSTRACT
1. Introduction
During their lifetime, dental composite restorations are exposed to an array of environmental factors involved in the breakdown of the adhesive interface between the restorative material and the dental tissues. For instance, when the polymerization shrinkage stress of a dental composite exceeds the interfacial bond strength, delamination and gap formation at the interface occurs [1–3]. These gaps are susceptible to infiltration by oral fluids, acids, metabolites and colonization by oral bacteria [4–6]. Even if the initial shrinkage stress is not sufficiently high to cause delamination, the mechanical, chemical and biological stresses from oral functions can contribute to interfacial degradation [7]. Ultimately, the loss of the marginal sealing facilitates oral bacteria accumulation at the interface, which may lead to secondary caries around composite restorations.
Masticatory forces from normal chewing and para-functional habits can impose great stress at the adhesive interface. Although the mechanical degradation of dental composite materials has been largely characterized [7–10], much less is known about the effect of mechanical challenge on the interfacial integrity of dental restorations. The few studies published so far show that cyclic loading leads to mechanical weakening of the adhesive interface regardless of the fatigue configuration (i.e. tensile, bending or compression) [11–13]. Moreover, similar to the fracture strength of monolithic materials, an inverse correlation between number of loading cycles and bond strength values has been shown [12, 14]. These data suggest that cyclic mechanical loading can affect the integrity of the bond irrespective of the initial bond strength of the adhesive interface. Thus, fatigue is relevant when exploring interfacial degradation processes.
In addition to mechanical challenge, biological and chemical agents present in oral fluids and food can affect dental composite restorations. Research by Santerre and coworkers demonstrated that esterase activity of saliva promotes the breakdown of condensation bonds present in dental composite polymers [15–19]. Those studies focused on the composite material, however, biological degradation effects on the adhesive interface remain largely unknown. A recent study showed that exposure of model restorations to esterases increased the amount of microbial leakage along the interface [20], suggesting that biological degradation could contribute to the interfacial breakdown.
Oral biofilms have been proposed to have a role in the degradation of the interface [21]. Recent data demonstrated that Streptococcus mutans, an oral pathogen, possesses esterase activity capable of degrading dental composites and dental adhesives [22] implying a potential microbial mechanism for degrading the adhesive interface.
In the mouth, oral biofilms produce organic acids by fermenting carbohydrates from diet [23], thus promoting the demineralization of hard dental tissues [24]. In addition, oral bacteria secrete a large array of enzymes and other metabolic products that might degrade the adhesive interface. To gain insight into the role of oral bacteria in the degradation process, we previously investigated the effect of a multi-species oral microcosm biofilm on the interfacial integrity of model dental restorations [25]. In that model, dentin-composite disks prepared with two different restorative systems were exposed to oral biofilms, and then tested under diametral compression. During the microbial challenge, half of the specimens were exposed to a sucrose-pulsed biofilm to explore the contribution of sucrose as an environmental factor. A reduction in the debonding load was observed in both groups exposed to biofilm compared to the control (no biofilm), although only the reduction seen in the sucrose-fed biofilm group was statistically significant.
Most of the factors involved in interfacial breakdown are commonly studied in isolation. In the oral cavity, multiple challenging factors may be present at the same time, and they might contribute synergistically to the degradation process. The aim of this study was to examine the combined effect of mechanical loading and microbial challenge on the interfacial integrity of composite restorations placed in extracted teeth, and to explore whether the effects of those challenges were modulated by environmental factors, such as sucrose.
2. Materials and methods
2.1 Specimen preparation
De-identified extracted human third molars were collected from oral surgery clinics at the University of Minnesota and in the Minneapolis/Saint Paul metropolitan area. Only teeth free of caries, fractures and cracks were included. The molars were cleaned of soft tissues and hard deposits (when present), and stored in 1% thymol solution. Mesio-occlusal-distal (MOD) class II cavities were prepared using a modified flat end tapered burs (SS White Burs Inc., Lakewood, NY, USA). The dimensions of the cavities were ~2.5mm wide and ~2.5mm deep for the occlusal box, and ~3mm wide and ~1.5mm deep for the proximal box. All teeth were restored using Adper™ Single Bond Plus adhesive system (SB) and Z100™ Restorative (Z100) system (3M ESPE, St. Paul, MN, USA) (Table 1). Briefly, 35% phosphoric acid (Scotchbond etchant, 3M ESPE, St. Paul, MN, USA) was applied to dentin and enamel for 15s and rinsed with water. Gentle air-drying was used to avoid collagen collapse in dentin. Two consecutive coatings of SB were rubbed on the internal walls of the preparation and polymerized for 20s. The teeth were restored with 2mm thick increments of Z100. Each layer was polymerized for 20s with an Elipar™ S10 curing light (3M ESPE, St. Paul, MN, USA) operated at 1200 mW/cm The samples were polished after 24h of water storage. A finishing diamond stone was used to remove excess material from the restoration margin before polishing was conducted with Progloss™ One Step Composite Polishers (Kerr Corporation, Orange, CA, USA). A total of 54 MOD class-II composite restorations were made.
Table 1.
Compositions of composite and adhesive used for Class-II restorations (obtained from manufacturer’s data sheets (3M ESPE))
| Product | Composition | Batch number |
|---|---|---|
| Z100™ Restorative | Silane treated ceramic, triethylene glycol dimethacrylate (TEGDMA), bisphenol a diglycidyl ether dimethacrylate (BISGMA), 2-benzotriazolyl-4-methylphenol. | N649950 |
| Adper Single Bond Plus | Ethyl alcohol, silane treated silica (nanofiller), bisphenol a diglycidyl ether dimethacrylate (BISGMA), 2-hydroxyethyl methacrylate(HEMA), glycerol 1,3-dimethacrylate, copolymer of acrylic and itaconic acids, water, diurethane dimethacrylate (UDMA) diphenyliodonium hexafluorophosphate, ethyl 4-dimethyl aminobenzoate (EDMAB). | N561025 |
2.2 Fatigue (chewing simulation)
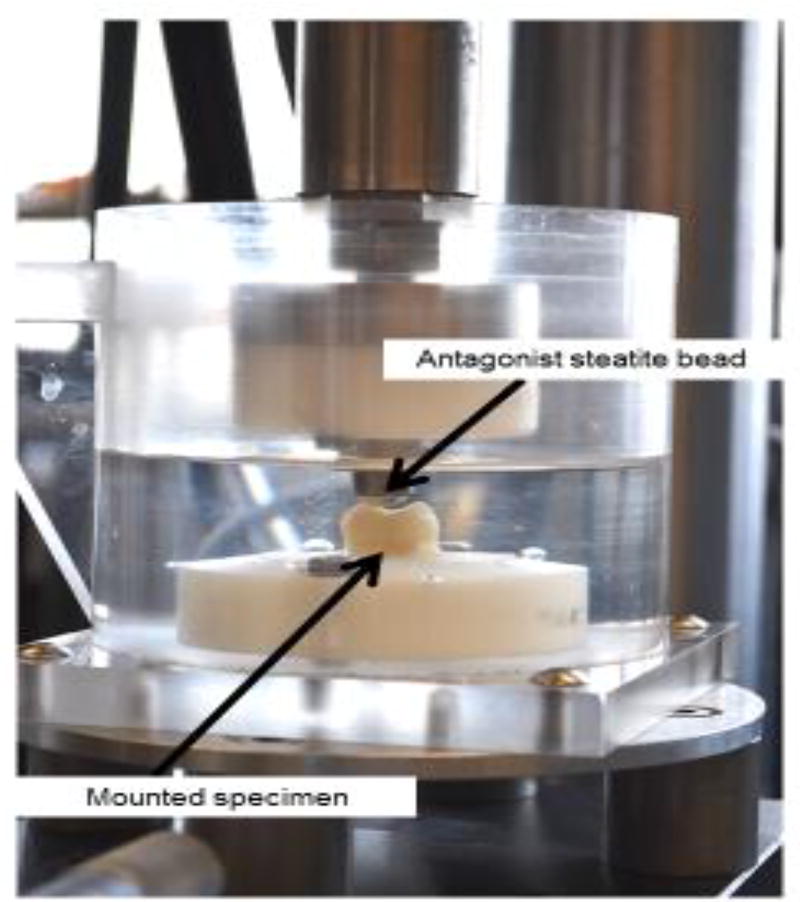
Half of the specimens (n=27) were mounted in Teflon rings with self-curing acrylic resin (Dentsply Caulk, Milford, DE, USA). Each mounted specimen was positioned in the mandibular chamber of an artificial oral environment (ART), developed by the Minnesota Dental Research Center for Biomaterials and Biomechanics [26] (Figure 1). A 6mm diameter steatite bead attached to the upper arm was used as the antagonist. The target loading point was located in the functional cusp of the third molars. The artificial mouth was set to simulate 200,000 chewing cycles with a maximum load of 50N and an excursion of 0.8mm at the contact point [27]. Throughout the duration of the mechanical challenge the specimens were submerged in deionized water at room temperature. The cyclic load was constantly monitored during the loading process.
Figure 1.
Cyclic loading setup. Each tooth was mounted in acrylic resin and located in the lower chamber of the artificial oral environment. A steatite bead attached to the upper loading arm acted as the antagonist.
2.3 Frozen multispecies biofilm stocks
Frozen multispecies biofilm stocks from multiple subjects were made and stored during the course of previous studies [25, 28, 29]. Briefly, plaque and saliva samples were collected from children with mixed dentitions (6–12 years of age) determined to be at high risk for caries. Plaque was retrieved from the margin of dental restorations. Whole saliva was collected by expectoration. Those sampling protocols were approved by the University of Minnesota Institutional Review Board; detailed descriptions are available in our previous publications [25, 28, 29].
This study design called for 12 sequential weeks of biofilm exposure. Our resources did not allow us to run stocks from multiple subjects. A pair of stocks from a single subject (781) were selected to provide the inoculums for the biofilm challenge in this study. One stock had been grown without sucrose (781NS), whereas the other had been pulsed with sucrose five times a day (781WS). The original plaque sample for subject 781 was collected from the margin of a composite restoration with active secondary caries. Detailed information on the taxonomic composition of the 781plaque sample, and the taxonomic and metaproteomic composition of the 781NS and 781WS biofilms can be found in the supplementary files that accompany a recent article [29].
2.4 In-vitro biofilm model
Before biofilm exposure, all the restored teeth (fatigued and non-fatigued) were coated with color-coded acid resistant nail varnish, leaving only the composite and the restoration margin exposed. The nail varnish was allowed to dry in a HEPA-filtered chamber, to minimize the likelihood of contamination. The two groups of specimens were further divided into three subgroups according to the biofilm growth condition in the experiment: Control (no biofilm), biofilm with no sucrose (BNS), and biofilm with sucrose (BWS). This defined six different groups (Table 2).
Table 2.
Specimen distribution per biofilm growth condition
| Control reactor (no biofilm) | -Fatigue Control |
| -No Fatigue Control | |
|
| |
| BNS reactor | -Fatigue BNS |
| -No Fatigue BNS | |
|
| |
| BWS reactor | -Fatigue BWS |
| -No Fatigue BWS | |
All of the following steps likewise were performed inside the HEPA enclosure. The color-coded samples were immersed in 75% ethanol for 1 min, air-dried, and then mounted in customized sample holders designed to fit in the sampling rods of the CDC reactor. The tooth-holder combinations were immersed in 75% ethanol for 1 min, and air-dried as before. They were then mounted in autoclaved sampling rods and the areas unprotected by nail varnish (composite and tooth interfaces) were coated with 30µl of filtered-sterilized saliva (originally collected from subject 781 and stored at −80°C). The specimens allocated to the BNS group were then inoculated with 30µl of 781NS biofilm stock re-suspended in anaerobic transport media. The BWS group was inoculated with a corresponding suspension of the 781WS stock.
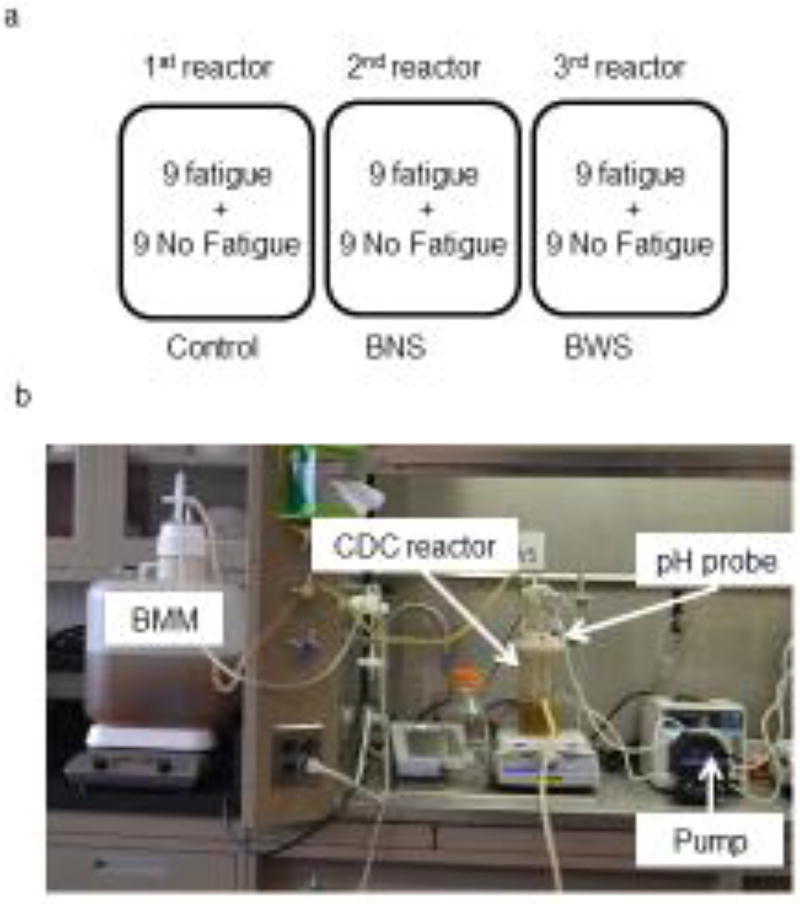
The rods with non-inoculated specimens (9 Fatigue and 9 No-fatigue) were inserted into the Control reactor, which contained 350 ml of sterile basal mucin medium (BMM) with 0.02% sodium azide added to inhibit microbial growth during the experiment (Figure 2a and b). The rest of the samples were allocated in the same manner to the BNS and BWS reactors, which each contained an initial volume of 350 ml sterile BMM without sodium azide (Figure 2a and b). A ~24-h incubation was carried out in all reactors at 37 °C under constant shear (125 rpm) but with no media flow. This allowed bacteria to attach to the restoration surface (in the case of the inoculated specimens). After incubation, the BNS and BWS reactors were connected to the nutrient carboy and sterile BMM was pumped through the system for 48 h (Figure 2b). The initial rate was set at 17 ml min−1 (125 rpm, 37 °C). In the BNS reactor, the biofilm was grown in the absence of sucrose, while in the BWS reactor sucrose was pulsed five times a day to simulate daily food intake. This was done by adding 42 ml of 40% sucrose (for a final concentration of 5%) into the reactor at intervals of 2 h. No sucrose pulsing was conducted at night. The flow rate was set at 20 ml min−1 during the second day of sucrose pulsing to avoid biofouling and to prevent plugging of the efflux tubing. BMM was not pumped through the Control reactor, in order to minimize the volume of sodium azide-containing hazardous waste produced. However, it was stirred continuously at 37 °C. After 48h the systems were taken down; the samples were cleaned by rinsing and sonication, disinfected with 75% ethanol, and stored in sterile phosphate-buffered saline at 4°C until the next biofilm challenge. Twelve sequential cycles of biofilm challenge with the same inoculum were conducted for the BNS and BWS teeth. During the same period, the Control teeth were exposed to fresh sterile BMM with sodium azide, as described above. Between each experimental cycle all instruments and containers used were disinfected with 10% bleach for 30 minutes, rinsed with water, washed with detergent, rinsed with water again and finally autoclaved prior to the next cycle. A fresh layer of nail varnish was applied before each run and the samples then were disinfected saliva-coated and inoculated as described above.
Figure 2.
(a) Diagram of the specimens’ allocation in each CDC reactor (b) CDC reactor setup for experimental runs with labeled components.
All reactors were incubated under aerobic conditions, to simulate the natural succession of supragingival plaque. Data published elsewhere had previously shown that anaerobic species were able to survive in this system, presumably due to oxygen consumption by facultative species [29].
2.4 pH recording
To continuously measure the pH changes in the medium, the CDC vessel’s lid was modified to fit an autoclavable pH electrode. The pH was recorded every 15 min throughout the 72-h period of experimentation.
2.5 Demineralization assessment using Micro-CT
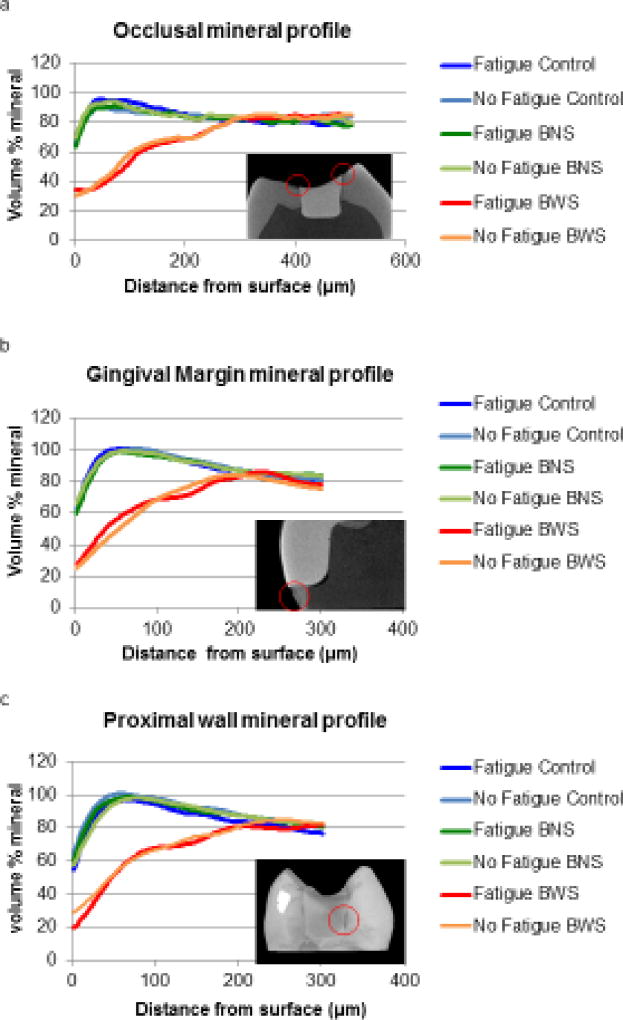
After 12 sequential biofilm challenges, three specimens from each group were scanned for demineralization around the interface using an X-ray micro-computed tomography (micro-CT) machine (XT H 225, Nikon Metrology, Brighton, MI, USA). The teeth were mounted with acrylic resin in Teflon rings and scanned with the following operational parameters: 90 kV, 90 µA, 720 projections and 4 frames per projection. 3D spatial reconstructions were done with CT Pro 3D (Nikon Metrology, Brighton, MI, USA) and visualized with VG Studio MAX 2.1 (Volume Graphics GmbH, Heidelberg, Germany). For the demineralization analysis, three different landmarks per sample were assessed: occlusal margin, proximal wall and gingival margin. For each chosen region, nine attenuation coefficient profiles were retrieved extending from the surface of the enamel lesion to the deeper sound enamel. The data from the three representative specimens were averaged and converted to mineral percentage profiles for each group.
2.6 Fracture test
Following the biofilm challenge, the restored teeth (including the scanned samples) were disinfected, and the protective layer of nail varnish was removed with a clean spatula. The teeth were then mounted in Teflon rings with acrylic resin with the occlusal plane facing up. Each specimen was fixed in the lower plate of a universal test machine (858 Mini Bionix II, MTS, MN, USA) and loaded until fracture with a stainless-steel hemispherical loading head (6mm in diameter). Loading was applied in a stroke-control mode at a cross-head speed of 0.1 mm min−1, and the maximum load achieved was taken to be the fracture load. The test specimens then were scanned with micro-CT to identify the fracture mode. Scanning parameters were: 90 kV, 90 µA, 720 projections and 2 frames per projection.
2.7 Statistical analysis
The mean fracture loads from the different groups were compared using two-way analysis of variance (ANOVA) with biofilm growth condition (Control, BNS, BWS) and fatigue (Fatigue, No Fatigue) as the between-group factors. Multiple comparisons among the different conditions were conducted with Bonferroni post hoc test at alpha level of 0.05.
3. Results
3.1 Sucrose pulsing of multispecies biofilm reduces pH in a CDC bioreactor
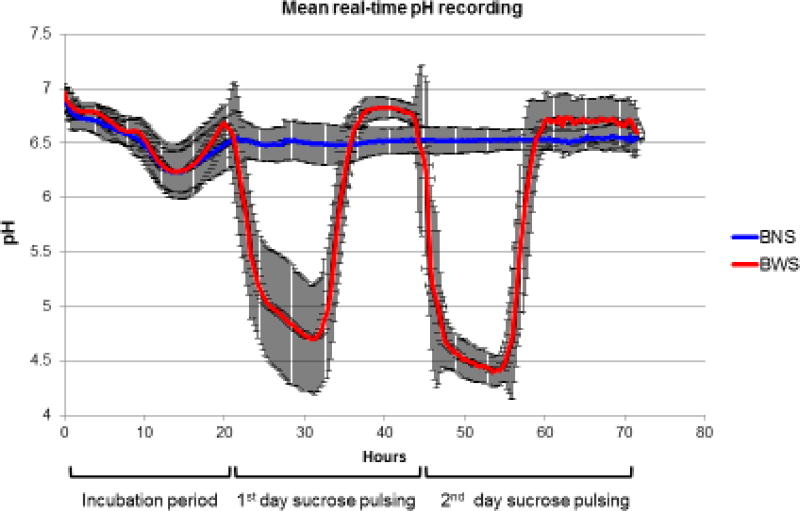
The pH in the medium was tracked over the duration of each experiment to study the effect of pulsing sucrose. Similar pH curves were observed throughout the twelve cycles of biofilm challenge. Real-time pH measurements were recorded from the CDC reactors containing samples in the presence of biofilm with and without sucrose pulsing (biofilm with sucrose, BWS; and, biofilm with no sucrose, BNS). The pH of the control group was not recorded, as it was known from previous experiments that it would remain at approximately 7.0 throughout the 72h incubation cycle. In the BWS condition the pH values drastically decreased after sucrose pulsing began. The pH remained at levels below the critical value for enamel (5.5) during sucrose pulsing, and returned to approximately 6.5 overnight, when sucrose pulsing stopped. By contrast, the pH in the BNS reactor remained between 6.5 and 7 throughout the period of media flow. Figure 3 shows the mean pH values for both groups, averaged over all 12 cycles. Those results indicated that sucrose pulsing was required to induce biofilm-mediated reductions in pH in the CDC bioreactor system.
Figure 3.
Mean real-time pH recording from the twelve-biofilm challenges for BNS and BWS growth conditions. Black bars indicate the standard deviation at each time point.
3.2 Enamel demineralization after 12 cycles of biofilm challenge
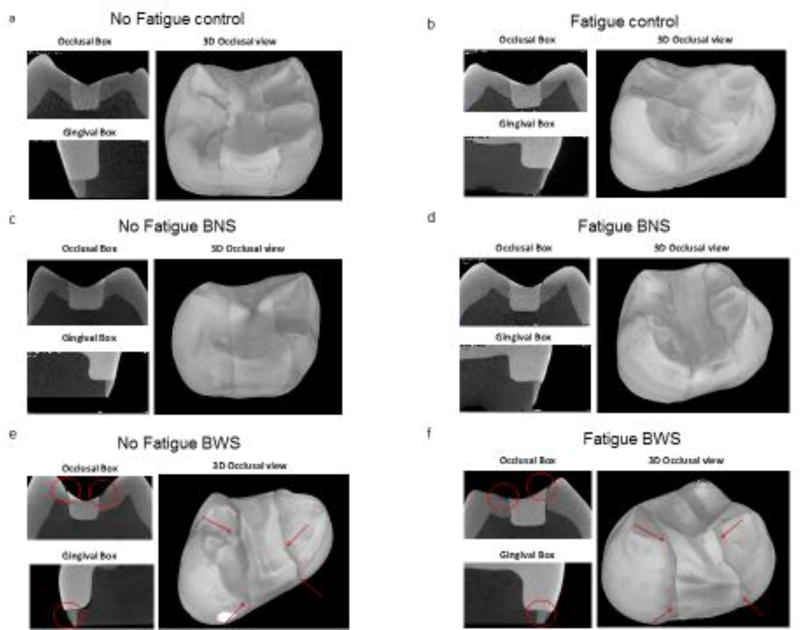
Three specimens from each experimental group were analyzed with micro-CT. The cross-sectional and 3D analysis showed no detectable demineralization in all the teeth under control and BNS conditions, with or without fatigue (Figure 4a, b, c and d). In those groups, the enamel surrounding the restorations was intact, and the only notable enamel degradation was limited to wear facets at the contact point of mechanical challenge in the experimental groups subjected to fatigue. In stark contrast, micro-CT images of BWS teeth, with or without fatigue, showed enamel demineralization in the areas next to the restoration margins that were not covered by nail varnish (Figure4e and 4f). There were no major differences in the degree of enamel demineralization surrounding the restoration margins between the fatigued and non-fatigued teeth. The enamel demineralization had the pattern of an outer lesion without the presence of a wall lesion. Overall, the areas of demineralized enamel were parallel to the enamel surface, and mostly uniform in thickness. Mineral percentage profiles taken from the occlusal surface, proximal wall and gingival margin showed that demineralization was around 200µm deep, regardless of the surface and the fatigue condition (Figure 5a,b and c), although it tended to be slightly deeper at the occlusal surface for both groups (Figure 5c). In some of the specimens where the enamel was thin or lost in the cervical region, demineralization of the gingival floor reached the underlying dentin (data not shown).
Figure 4.
Representative micro-CT cross-sectional images and 3D reconstructions of the restored teeth after fatigue and biofilm challenge. (a) No Fatigue control (no biofilm) (b) Fatigue control (no biofilm). (c) No Fatigue BNS. (d) Fatigue BNS (e) No Fatigue BWS. (f) Fatigue BWS. Red circles and red arrows indicate zones with observed demineralization in the 2D cross-sections and 3D reconstructions, respectively.
Figure 5.
Average mineral profiles obtained from three different regions in the teeth for each of the groups. (a) occlusal margin (b) gingival margin, and (c) proximal wall. Inset in each plot is a representative image indicating the approximate locations from where the profiles were obtained.
3.3 Fracture loads are significantly reduced by sucrose pulsed biofilms independent of fatigue challenge
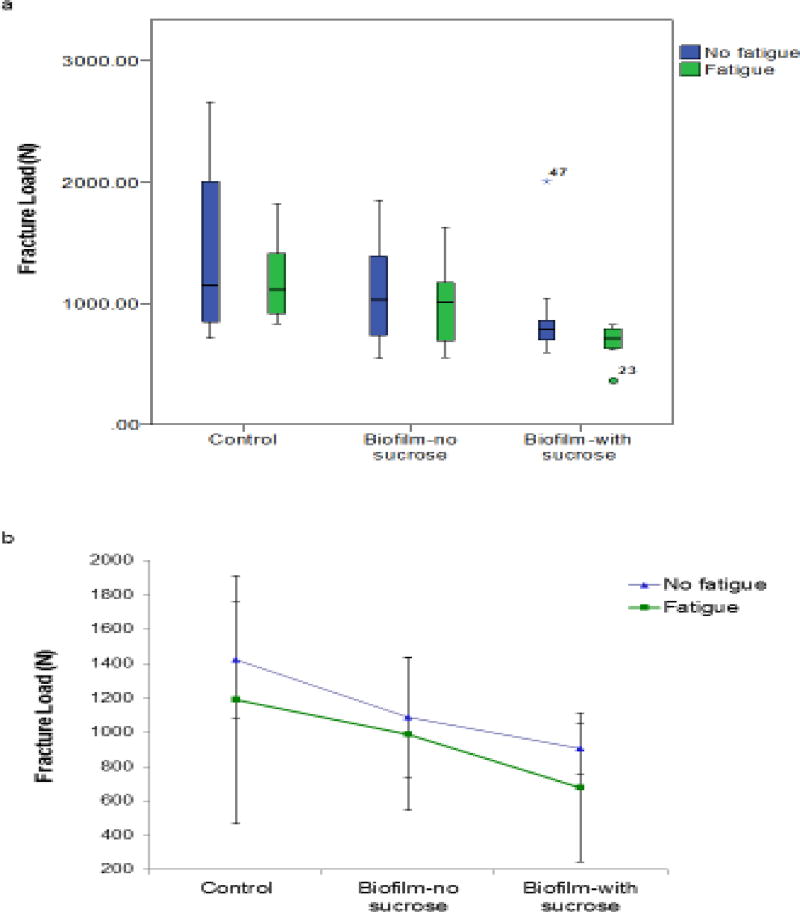
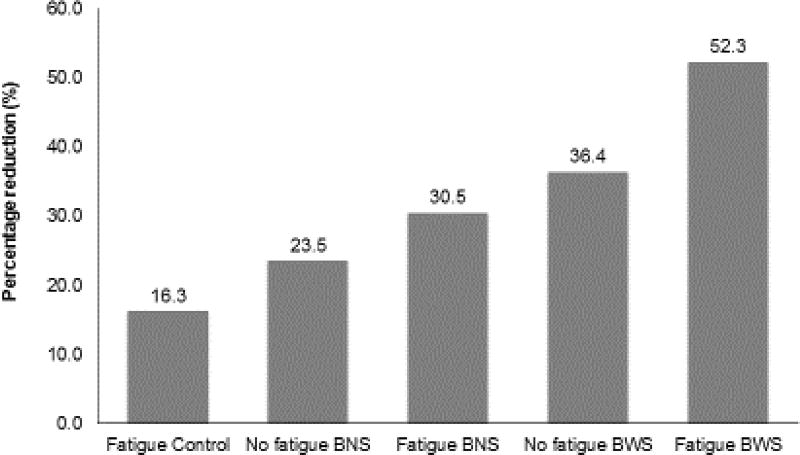
To study the effect of fatigue and biofilm exposure on interfacial degradation, the specimens were subjected to fracture test after mechanical and biofilm challenges. Table 2 summarizes the mean fracture loads for each group and condition. In general, fatigued samples displayed slightly lower fracture loads than non-fatigued specimens (Table 3, Figure 6a and b). However, the effect of fatigue was not statistically significant, even though the fatigued groups consistently trended lower (p = 0.149). When the biofilm growth conditions were considered, the control groups showed the highest fracture loads followed by BNS and BWS, for both Fatigue and No Fatigue groups. The effect of biofilm was significant (p = 0.007). Bonferroni t-tests indicated that the fracture load reduction in BWS was statistically significant (p = 0.006). Although the profile plot (Figure 6b) suggested a consistent trend, BNS was not significantly different from either BWS or control by the Bonferroni test. To explore the combined effect of fatigue and biofilm, the percentage of load reduction from each group was calculated relative to the mean fracture load of the No Fatigue Control group (Fig. 7). The results showed that fatigue alone generated a reduction of ~16%, whereas No Fatigue BNS and No Fatigue BWS displayed a reduction of ~23.5% and ~36.4%, respectively. The reduction generated by adding fatigue to BNS (30.5%) and BWS (52.4%) suggested that the combined effects of fatigue and biofilm might reflect an additive trend, even though the fatigue effect was not statistically significant overall.
Table 3.
Mean and standard deviation of fracture loads (N) by group and condition
| No fatigue (N) | Fatigue (N) | |
|---|---|---|
| Control | 1422.4 ± 722.7 | 1190.6 ± 340.1 |
| BNS | 1087.6 ± 444.6 | 988.8 ± 353.4 |
| BWS | 904.3 ± 433.1 | 678.9 ± 149.5 |
Figure 6.
Data distribution and mean profile of fracture loads: a) Box plots displaying the median fracture load (black bar dividing each box) for each group and condition. Each box represents the inter-quartile range. End of whiskers are the minimum and maximum load values (except for outliers). b) Profile plots presenting the mean fracture load values and standard deviation for each group and condition.
Figure 7.
Fracture load reduction of each group relative to non-fatigued Control group. The magnitude of fracture load reduction by adding fatigue challenge to BNS and BWS suggests only a modest additive effect.
Three main modes of fracture were found: interfacial, cohesive in dental tissue or composite, and mixed mode (Interfacial and cohesive). In general, the presence of biofilm seemed to produce more cohesive failures in enamel compared to their respective controls in both fatigued and non-fatigued groups. However, no clear trend was observed between failure mode and the different conditions.
4. Discussion
Dental composite restorations fail more frequently than those of amalgams, because of higher instances of secondary caries [30, 31]. Breakdown of interfacial integrity can occur from the first moment that a dental composite is placed in the cavity preparation. Shrinkage stress can lead to the formation of micro-spaces at the interface allowing passage of oral fluids and bacteria [4]. Mechanical challenges from mastication or para-functional habits can perpetuate the damage to the interface by inducing mechanical weakening of the adhesive bond [11–13]. Moreover, chemical and biological challenges can also affect the interfacial integrity of the composite restorations [15, 16, 21]. These factors, however, are usually studied in isolation making it difficult to determine their relative contribution to the process of degradation. Here, we developed a more comprehensive model to study the combined effect of mechanical and microbial challenge on the degradation of the adhesive interface.
A multispecies biofilm model was chosen to provide a more realistic representation of oral microbial diversity. For many years, research has focused on Streptococcus mutans. Although valuable knowledge has been obtained, it is currently estimated that the oral microbiome encompasses over 600 species [32]. In an earlier study using a CDC reactor and basal mucin medium, we were able to grow reproducible oral microcosm biofilms that retained 60% of the original inoculum at the species level. Furthermore, it was found that multispecies oral biofilms induced interfacial degradation in a simple dentin-ring composite restoration model [25]. Here, this CDC reactor biofilm model was combined with mechanical cycling of class-II restorations in extracted teeth, in an artificial oral environment that simulates chewing cycles.
Class-II restorations involve removal of a larger amount of the tooth. That reduces the strength of the teeth [33, 34], and leads to higher failure rates [35–37]. High shear and tensile stresses concentrate at the interface of direct composite MOD restorations when subjected to chewing simulations, which can threaten interfacial integrity [38]. Even though interfacial degradation and secondary caries can develop anywhere in the tooth, studies have reported that secondary caries mostly occurs at gingival margin of class-II and class-V restorations [39–41]. This is probably due to the higher plaque accumulation at these sites, incomplete adaptation of the materials to the restoration margin, and poor adhesion [40, 41]. Therefore, a class-II composite restoration provided a suitable model to study the combined effects of mechanical loading and microbial challenge.
Dental restorations rarely fail catastrophically under a single application of biting force. Instead, they fail due to repeated loading with force levels that are unlikely to generate catastrophic failures after just one application [42, 43]. Therefore, fatigue challenge is a better tool to understand the in-vivo behavior of adhesive systems when subjected to forces. In our study, we simulated chewing cycles using an artificial oral environment. We used 200,000 cycles in order to simulate ~8 months of masticatory function, which was followed by biofilm exposure to explore potential synergistic effects between mechanical and microbial challenge. According to the percentage of fracture load reduction, Fatigue BNS and Fatigue BWS showed greater load reduction compared to Fatigue Control, No Fatigue BNS, or No Fatigue BWS, but the magnitude of the drop suggested only a modest additive combined effect between fatigue and the presence of biofilm (Fig. 7).
The presence of biofilm led to a more pronounced reduction in fracture loads. When the results were averaged over the Fatigue and No Fatigue groups, the fracture loads decreased by 270N in the case of BNS and around 515N in the case of BWS compared to the control groups that were not exposed to oral biofilm. Further analysis showed that only the differences between BWS and the control group were statistically significant, suggesting that sucrose pulsing greatly enhanced any initial biofilm effect.
Most of the research investigating mechanical degradation of the adhesive interface has used small specimens of the adhesive interface, such as the matchstick specimens in micro-tensile tests [11, 13, 27]. It is probable that the strong effect of fatigue shown in those studies is less pronounced in a whole restoration, because it is more damage tolerant due to redundancy. Others have shown a strong effect of cyclic loading on the marginal integrity of class-II composite restorations, where mechanical loading decreased significantly the number of gap free surfaces around the restorations [27, 44]. Though we did not test for marginal gap formation in this study, a reduction in the fracture loads was expected if gaps were formed or enlarged during masticatory challenge. Such an effect was seen in the Control group for this study, but not to a great extent.
In our analysis, we observed a great reduction in pH in the presence of biofilm with sucrose pulsing. This reduction reached values lower than the critical pH value for enamel (~5.5) [45], and much lower than the critical pH for dentin (pH~6.5) [46]. This explains why enamel demineralization was found in both Fatigue and No Fatigue BWS teeth. It was expected that the levels of demineralization would be more pronounced in the Fatigue BWS teeth, due to added interfacial breakdown and more penetration of bacteria. However, micro-CT analysis did not show major differences between the two groups. Several reasons could explain this. First, it is possible that additional demineralization in the fatigued teeth could have been below the detection threshold for micro-CT. Micro-CT is based on the X-ray attenuation properties of materials. Consequently, demineralization changes that occurred only at the ultrastructural level without changing enamel density might not be detected. A second possibility is that the fatigue used in this study was insufficient to create or enlarge marginal gaps enough to allow bacteria passage and thus did not promote additional demineralization along the interface. The fatigue challenge was done at 200,000 cycles which equates to approximately 8 months of chewing [47], whereas the biofilm challenge is estimated to represent between 6 months to one year, based on the depth of demineralization reached (Fig. 5). A third possibility is that the demineralization event happens independently of the mechanical breakdown of the interface, which would support the idea that secondary caries is a primary lesion next to a dental restoration driven by plaque accumulation, accompanied by a cariogenic diet. Indeed, micro-CT and optical microscopy (data not shown) showed demineralization patterns of an outer lesion with no signs of wall lesion formation, even though the restorative material used here (Z100) has displayed high levels of microleakage [48]. This would support the argument that the presence of microleakage is less important for secondary caries formation compared to the accumulation of a cariogenic oral biofilm [49]. However, it is possible that the number of cycles between biofilm and fatigue were not enough to generate a wall lesion, or that it is needed to conduct the two challenges simultaneously, as occurs clinically. We were not able to accomplish that using our current model, but future research may lead to development of a combined system.
For these experiments we were able to use paired biofilm stocks from only a single subject for all 12 non-sucrose and sucrose challenges. Because of that limitation, it is important to be cautious in generalizing the results to other individuals. That said, a drastic pH reduction was only seen in the presence of sucrose pulsing, and the same pattern was characteristic in our previous biofilm reactor studies with inoculums from other subjects [25, 28, 29]. This highlights the importance of environmental factors in inducing dysbiosis of oral biofilms. Some authors have suggested that other acidogenic and acid tolerant bacteria besides mutans streptococci might be associated with dental caries [50, 51], and others have shown similar microbial composition between caries active and caries free subjects [52]. Interestingly, next generation sequencing16S rRNA analysis detected only low levels of S. mutans in the original plaque inoculum from subject 781 (0.5% of total reads), while S. mutans (if present) was below the threshold of detection in the 781NS and 781WS microcosm biofilms [29]. Moreover, recent studies have shown conserved metabolic functions in taxonomically diverse oral biofilm communities associated with disease [29, 53], suggesting that sugar-induced dysbiosis of oral biofilm will be more strongly associated with caries than any specific group of oral bacteria.
5. Conclusions
In summary, we have presented a more comprehensive model to study the effects of mechanical and microbial challenge on the interfacial integrity of dental restorations. The results showed that when specimens are subjected to both challenges, a greater reduction in fracture strength is seen compared to the effects of fatigue alone or biofilm alone suggesting an additive effect. The presence of biofilm induced a larger reduction in fracture strength, which became significant in the presence of sucrose pulsing. Overall, the effect of fatigue was much less pronounced.
Acknowledgments
This study was partially supported by the National Institute of Dental and Craniofacial Research (NIDCR), USA through Grant No. 1 R01 DE021366, the CONICYT Becas Chile Scholarship Program, Chilean Government and a Doctoral Dissertation fellowship, University of Minnesota. In addition, we would like to thank Dr. Robert S. Jones for his contributions to this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors of this manuscript declare that no benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1.Boaro LC, Froes-Salgado NR, Gajewski VE, Bicalho AA, Valdivia AD, Soares CJ, et al. Correlation between polymerization stress and interfacial integrity of composites restorations assessed by different in vitro tests. Dent Mater. 2014;30:984–92. doi: 10.1016/j.dental.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto T, Ferracane JL, Sakaguchi RL, Swain MV. Calculation of contraction stresses in dental composites by analysis of crack propagation in the matrix surrounding a cavity. Dent Mater. 2009;25:543–50. doi: 10.1016/j.dental.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto T, Nishide A, Swain MV, Ferracane JL, Sakaguchi RL, Momoi Y. Contraction stresses in dental composites adjacent to and at the bonded interface as measured by crack analysis. Acta Biomater. 2011;7:417–23. doi: 10.1016/j.actbio.2010.07.040. [DOI] [PubMed] [Google Scholar]
- 4.Cenci MS, Pereira-Cenci T, Cury JA, Ten Cate JM. Relationship between gap size and dentine secondary caries formation assessed in a microcosm biofilm model. Caries Res. 2009;43:97–102. doi: 10.1159/000209341. [DOI] [PubMed] [Google Scholar]
- 5.Diercke K, Lussi A, Kersten T, Seemann R. Isolated development of inner (wall) caries like lesions in a bacterial-based in vitro model. Clin Oral Investig. 2009;13:439–44. doi: 10.1007/s00784-009-0250-z. [DOI] [PubMed] [Google Scholar]
- 6.Totiam P, Gonzalez-Cabezas C, Fontana MR, Zero DT. A new in vitro model to study the relationship of gap size and secondary caries. Caries Res. 2007;41:467–73. doi: 10.1159/000107934. [DOI] [PubMed] [Google Scholar]
- 7.Drummond JL. Degradation, fatigue, and failure of resin dental composite materials. J Dent Res. 2008;87:710–9. doi: 10.1177/154405910808700802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Indrani DJ, Cook WD, Televantos F, Tyas MJ, Harcourt JK. Fracture toughness of water-aged resin composite restorative materials. Dent Mater. 1995;11:201–7. doi: 10.1016/0109-5641(95)80019-0. [DOI] [PubMed] [Google Scholar]
- 9.Lohbauer U, von der Horst T, Frankenberger R, Kramer N, Petschelt A. Flexural fatigue behavior of resin composite dental restoratives. Dent Mater. 2003;19:435–40. doi: 10.1016/s0109-5641(02)00088-x. [DOI] [PubMed] [Google Scholar]
- 10.Willems G, Lambrechts P, Braem M, Celis JP, Vanherle G. A classification of dental composites according to their morphological and mechanical characteristics. Dent Mater. 1992;8:310–9. doi: 10.1016/0109-5641(92)90106-m. [DOI] [PubMed] [Google Scholar]
- 11.Belli R, Baratieri LN, Braem M, Petschelt A, Lohbauer U. Tensile and bending fatigue of the adhesive interface to dentin. Dent Mater. 2010;26:1157–65. doi: 10.1016/j.dental.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Frankenberger R, Pashley DH, Reich SM, Lohbauer U, Petschelt A, Tay FR. Characterisation of resin-dentine interfaces by compressive cyclic loading. Biomaterials. 2005;26:2043–52. doi: 10.1016/j.biomaterials.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Staninec M, Kim P, Marshall GW, Ritchie RO, Marshall SJ. Fatigue of dentin-composite interfaces with four-point bend. Dent Mater. 2008;24:799–803. doi: 10.1016/j.dental.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Carrera C, Chen R, Li J, Chen Y, Lenton P, et al. Fatigue failure of dentin-composite disks subjected to cyclic diametral compression. Dent Mater. 2015;31:778–88. doi: 10.1016/j.dental.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaffer F, Finer Y, Santerre JP. Interactions between resin monomers and commercial composite resins with human saliva derived esterases. Biomaterials. 2002;23:1707–19. doi: 10.1016/s0142-9612(01)00298-8. [DOI] [PubMed] [Google Scholar]
- 16.Lin BA, Jaffer F, Duff MD, Tang YW, Santerre JP. Identifying enzyme activities within human saliva which are relevant to dental resin composite biodegradation. Biomaterials. 2005;26:4259–64. doi: 10.1016/j.biomaterials.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Santerre JP, Shajii L, Leung BW. Relation of dental composite formulations to their degradation and the release of hydrolyzed polymeric-resin-derived products. Crit Rev Oral Biol Med. 2001;12:136–51. doi: 10.1177/10454411010120020401. [DOI] [PubMed] [Google Scholar]
- 18.Santerre JP, Shajii L, Tsang H. Biodegradation of commercial dental composites by cholesterol esterase. J Dent Res. 1999;78:1459–68. doi: 10.1177/00220345990780081201. [DOI] [PubMed] [Google Scholar]
- 19.Shajii L, Santerre JP. Effect of filler content on the profile of released biodegradation products in micro-filled bis-GMA/TEGDMA dental composite resins. Biomaterials. 1999;20:1897–908. doi: 10.1016/s0142-9612(99)00087-3. [DOI] [PubMed] [Google Scholar]
- 20.Kermanshahi S, Santerre JP, Cvitkovitch DG, Finer Y. Biodegradation of resin-dentin interfaces increases bacterial microleakage. J Dent Res. 2010;89:996–1001. doi: 10.1177/0022034510372885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delaviz Y, Finer Y, Santerre JP. Biodegradation of resin composites and adhesives by oral bacteria and saliva: a rationale for new material designs that consider the clinical environment and treatment challenges. Dent Mater. 2014;30:16–32. doi: 10.1016/j.dental.2013.08.201. [DOI] [PubMed] [Google Scholar]
- 22.Bourbia M, Ma D, Cvitkovitch DG, Santerre JP, Finer Y. Cariogenic bacteria degrade dental resin composites and adhesives. J Dent Res. 2013;92:989–94. doi: 10.1177/0022034513504436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koo H, Falsetta ML, Klein MI. The exopolysaccharide matrix: a virulence determinant of cariogenic biofilm. J Dent Res. 2013;92:1065–73. doi: 10.1177/0022034513504218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marsh PD. Microbiology of dental plaque biofilms and their role in oral health and caries. Dent Clin North Am. 2010;54:441–54. doi: 10.1016/j.cden.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Carrera C, Chen R, Li J, Lenton P, Rudney JD, et al. Degradation in the dentin-composite interface subjected to multi-species biofilm challenges. Acta Biomater. 2013 doi: 10.1016/j.actbio.2013.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeLong R, Douglas WH. An artificial oral environment for testing dental materials. IEEE Trans Biomed Eng. 1991;38:339–45. doi: 10.1109/10.133228. [DOI] [PubMed] [Google Scholar]
- 27.Frankenberger R, Strobel WO, Kramer N, Lohbauer U, Winterscheidt J, Winterscheidt B, et al. Evaluation of the fatigue behavior of the resin-dentin bond with the use of different methods. J Biomed Mater Res B Appl Biomater. 2003;67:712–21. doi: 10.1002/jbm.b.10052. [DOI] [PubMed] [Google Scholar]
- 28.Rudney JD, Chen R, Lenton P, Li J, Li Y, Jones RS, et al. A reproducible oral microcosm biofilm model for testing dental materials. J Appl Microbiol. 2012;113:1540–53. doi: 10.1111/j.1365-2672.2012.05439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudney JD, Jagtap PD, Reilly CS, Chen R, Markowski TW, Higgins L, et al. Protein relative abundance patterns associated with sucrose-induced dysbiosis are conserved across taxonomically diverse oral microcosm biofilm models of dental caries. Microbiome. 2015;3:69. doi: 10.1186/s40168-015-0136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernardo M, Luis H, Martin MD, Leroux BG, Rue T, Leitao J, et al. Survival and reasons for failure of amalgam versus composite posterior restorations placed in a randomized clinical trial. J Am Dent Assoc. 2007;138:775–83. doi: 10.14219/jada.archive.2007.0265. [DOI] [PubMed] [Google Scholar]
- 31.Soncini JA, Maserejian NN, Trachtenberg F, Tavares M, Hayes C. The longevity of amalgam versus compomer/composite restorations in posterior primary and permanent teeth: findings From the New England Children's Amalgam Trial. J Am Dent Assoc. 2007;138:763–72. doi: 10.14219/jada.archive.2007.0264. [DOI] [PubMed] [Google Scholar]
- 32.Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, et al. The human oral microbiome. J Bacteriol. 2010;192:5002–17. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larson TD, Douglas WH, Geistfeld RE. Effect of prepared cavities on the strength of teeth. Oper Dent. 1981;6:2–5. [PubMed] [Google Scholar]
- 34.Mondelli J, Sene F, Ramos RP, Benetti AR. Tooth structure and fracture strength of cavities. Braz Dent J. 2007;18:134–8. doi: 10.1590/s0103-64402007000200009. [DOI] [PubMed] [Google Scholar]
- 35.Opdam NJ, Bronkhorst EM, Roeters JM, Loomans BA. A retrospective clinical study on longevity of posterior composite and amalgam restorations. Dent Mater. 2007;23:2–8. doi: 10.1016/j.dental.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 36.Opdam NJ, van de Sande FH, Bronkhorst E, Cenci MS, Bottenberg P, Pallesen U, et al. Longevity of posterior composite restorations: a systematic review and meta-analysis. J Dent Res. 2014;93:943–9. doi: 10.1177/0022034514544217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Nieuwenhuysen JP, D'Hoore W, Carvalho J, Qvist V. Long-term evaluation of extensive restorations in permanent teeth. J Dent. 2003;31:395–405. doi: 10.1016/s0300-5712(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 38.Dejak B, Mlotkowski A. A comparison of stresses in molar teeth restored with inlays and direct restorations, including polymerization shrinkage of composite resin and tooth loading during mastication. Dent Mater. 2015;31:e77–87. doi: 10.1016/j.dental.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 39.Mjor IA. Frequency of secondary caries at various anatomical locations. Oper Dent. 1985;10:88–92. [PubMed] [Google Scholar]
- 40.Mjor IA. The location of clinically diagnosed secondary caries. Quintessence Int. 1998;29:313–7. [PubMed] [Google Scholar]
- 41.Mjor IA. Clinical diagnosis of recurrent caries. J Am Dent Assoc. 2005;136:1426–33. doi: 10.14219/jada.archive.2005.0057. [DOI] [PubMed] [Google Scholar]
- 42.da cunha Mello FS, Feilzer AJ, de Gee AJ, Davidson CL. Sealing ability of eight resin bonding systems in a Class II restoration after mechanical fatiguing. Dent Mater. 1997;13:372–6. doi: 10.1016/s0109-5641(97)80109-1. [DOI] [PubMed] [Google Scholar]
- 43.Dewji HR, Drummond JL, Fadavi S, Punwani I. Bond strength of Bis-GMA and glass ionomer pit and fissure sealants using cyclic fatigue. Eur J Oral Sci. 1998;106:594–9. doi: 10.1046/j.0909-8836.1998.eos106110.x. [DOI] [PubMed] [Google Scholar]
- 44.Aggarwal V, Logani A, Jain V, Shah N. Effect of cyclic loading on marginal adaptation and bond strength in direct vs. indirect class II MO composite restorations. Oper Dent. 2008;33:587–92. doi: 10.2341/07-152. [DOI] [PubMed] [Google Scholar]
- 45.Margolis HC, Zhang YP, Lee CY, Kent RL, Jr, Moreno EC. Kinetics of enamel demineralization in vitro. J Dent Res. 1999;78:1326–35. doi: 10.1177/00220345990780070701. [DOI] [PubMed] [Google Scholar]
- 46.Hoppenbrouwers PM, Driessens FC, Borggreven JM. The mineral solubility of human tooth roots. Arch Oral Biol. 1987;32:319–22. doi: 10.1016/0003-9969(87)90085-9. [DOI] [PubMed] [Google Scholar]
- 47.DeLong R, Pintado MR, Douglas WH, Fok AS, Wilder AD, Jr, Swift EJ, Jr, et al. Wear of a dental composite in an artificial oral environment: A clinical correlation. J Biomed Mater Res B Appl Biomater. 2012;100:2297–306. doi: 10.1002/jbm.b.32801. [DOI] [PubMed] [Google Scholar]
- 48.Carrera CA, Lan C, Escobar-Sanabria D, Li Y, Rudney J, Aparicio C, et al. The use of micro-CT with image segmentation to quantify leakage in dental restorations. Dent Mater. 2015 doi: 10.1016/j.dental.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kidd EA, Fejerskov O. What constitutes dental caries? Histopathology of carious enamel and dentin related to the action of cariogenic biofilms. J Dent Res. 2004;83(C):C35–8. doi: 10.1177/154405910408301s07. [DOI] [PubMed] [Google Scholar]
- 50.Jiang W, Zhang J, Chen H. Pyrosequencing analysis of oral microbiota in children with severe early childhood dental caries. Curr Microbiol. 2013;67:537–42. doi: 10.1007/s00284-013-0393-7. [DOI] [PubMed] [Google Scholar]
- 51.Kanasi E, Dewhirst FE, Chalmers NI, Kent R, Jr, Moore A, Hughes CV, et al. Clonal analysis of the microbiota of severe early childhood caries. Caries Res. 2010;44:485–97. doi: 10.1159/000320158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang M, Chen Y, Xie L, Li Y, Jiang H, Du M. Pyrosequencing of Plaque Microflora In Twin Children with Discordant Caries Phenotypes. PLoS One. 2015;10:e0141310. doi: 10.1371/journal.pone.0141310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jorth P, Turner KH, Gumus P, Nizam N, Buduneli N, Whiteley M. Metatranscriptomics of the human oral microbiome during health and disease. MBio. 2014;5:e01012–14. doi: 10.1128/mBio.01012-14. [DOI] [PMC free article] [PubMed] [Google Scholar]