Abstract
Cardiac stem cells (CSCs) are important for improving cardiac function following myocardial infarction, with CSC migration to infarcted or ischemic myocardium important for cardiac regeneration. Strategies to improve cell migration may improve the efficiency of myocardial regeneration. Basic fibroblast growth factor (bFGF) is an essential molecule in cell migration, but the endogenous bFGF level is too low to be effective. The effect of exogenously delivered bFGF on CSC migration was observed in vitro and in vivo in the present study. The CSC migration index in response to various bFGF concentrations was demonstrated in vitro. In addition, a murine myocardial infarction model was constructed and bFGF protein expression levels and CSC aggregation following myocardial infarction were observed. To study cell migration in vivo, CM-Dil-labeled CSCs or bFGF-CSCs were injected into the peri-infarct myocardium following myocardium infarction and cell migration and maintenance in the peri-infarct/infarct area was observed 1 week later. Protein expression levels of bFGF, CXCR-4 and SDF-1 were assessed, as was myocardium capillary density. The Akt inhibitor deguelin was used to assess the role of the PI3K/Akt pathway in vitro and in vivo. The present study demonstrated that bFGF-promoted Sca-1+ CSC migration, with the highest migration rate occurring at a concentration of 45 ng/ml. The PI3K/Akt pathway inhibitor deguelin attenuated this increase. The phospho-Akt/Akt ratio was elevated significantly after 30 min of bFGF exposure. Transplantation of bFGF-treated Sca-1+ CSCs led to improved cell maintenance in the peri-infarct area and increased cell migration to the infarct area, as well as improved angiogenesis. Protein expression levels of bFGF, CXCR-4 and SDF-1 were upregulated, and this upregulation was partially attenuated by deguelin. Therefore, bFGF was demonstrated to promote Sca-1+ CSC migration both in vitro and in vivo, partially through activation of the PI3K/Akt pathway. This may provide a new method for facilitating CSC therapy for myocardium repair after myocardium injury.
Keywords: basic fibroblast growth factor, Sca-1 positive cardiac stem cells, cell migration
Introduction
Local ischemia and hypoxia following myocardial infarction results in the loss of cardiomyocytes and tissue damage (1). Stem cell therapy for myocardial regeneration is a promising treatment and the development of resident cardiac stem cell (CSC) therapy provides a novel option for treating myocardial infarction (2). Sca-1 (stem cells antigen-1) is a mouse protein that is commonly used to identify CSCs and these CSCs are capable of electrical coupling with local cardiomyocytes by differentiation into functional cardiomyocytes (3,4). Following myocardial infarction, CSCs migrate into the peri-ischemic and ischemic areas of the heart via cytokine-induced chemotaxis and participate in myocardial regeneration by differentiating into new cardiomyocytes, endothelial cells or smooth muscle cells (5–7).
The migratory ability of CSCs is important for successful implantation and subsequent amelioration of function during myocardial regeneration (8). Multiple signaling pathways participate in CSC migration and recruitment. The phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling pathway has been demonstrated to be essential for stem cell functions, including cell migration, cell survival and apoptosis (9). Stromal cell-derived factor 1 (SDF-1) is also a key factor in cardiac stem cell recruitment: By binding to a specific G protein coupled receptor on the cell membrane, C-X-C chemokine receptor type 4 (CXCR-4), SDF-1 induces chemotaxis and homing of several progenitor cells including CSCs, endothelial progenitor cells and mesenchymal stem cells (10). Elevated SDF-1 expression in the heart following infarction is correlated with increased CSC recruitment and improved myocardial regeneration (11). Successful migration of CSCs to the site of infarction or ischemic myocardium is important for cardiac regeneration, with poor migration of CSCs limiting the potential of cell therapy (12). Exogenous delivery of cell migration-enhancing cytokines may overcome this limitation and improve the efficacy of stem cell therapy for myocardial regeneration (13).
Basic fibroblast growth factor (bFGF) is an important molecule involved in heart remodeling and regeneration following myocardial infarction (14). In the myocardial regeneration process, bFGF stimulates the proliferation and migration of fibroblasts and participates in the formation of collagen to maintain heart function following loss of cardiomyocytes (15). bFGF may improve ventricular remodeling by regulating the relative proportions of collagen I and III (16). bFGF also stimulates the migration and proliferation of vascular endothelial cells and smooth muscle cells, and is viewed as a multipotent angiogenic stimulus that is important for tissue regeneration (17). Following myocardial infarction, the expression of bFGF in cardiomyocytes, vascular endothelial cells and smooth muscle cells in the ischemic and peri-ischemic area is elevated, improving cell migration, increasing the local blood supply and subsequently ameliorating cardiac function (18,19).
The adult human heart is recognized as a self-renewing organ as it contains its own native stem cells, CSCs (20). These CSCs are able to renew themselves, differentiating into new cardiomyocytes, endothelial cells and smooth muscle cells to replace injured or dead cells, under the stimulation of cytokines such as vascular endothelial growth factor (VEGF), bFGF and hypoxia-inducible factors (HIF) (4,5). Following myocardial infarction, activated CSCs migrate from their in situ residence and gather in ischemic and peri-ischemic areas to participate in tissue regeneration (8). Cell therapy based on CSCs provides a novel treatment for myocardial infarction and subsequent heart failure (21,22). There are three main subgroups of CSCs in the heart, of which the Sca-1+ group is the most numerous. It has previously been demonstrated that the number of Sca-1+ cells is ~100 fold greater than the other two subgroups. Sca-1+ CSCs display three central characteristics: Multipotency, self-renewal and clone formation ability (23).
There is previous evidence that in a murine myocardial infarction model, intra-myocardium injection of Sca-1+ CSCs reduced infarction size, attenuated left ventricular remodeling and ameliorated cardiac function (24). However, CSC therapy is limited by insufficient numbers of transplanted cells to replace the numerous lost cardiomyocytes (25,26). In order to solve this problem, it is important to induce resident CSC migration into the peri-ischemic and ischemic areas.
The present study aimed to investigate the effect of exogenously delivered bFGF on the migratory ability of CSCs in vitro and in vivo, which is important for CSC-based therapy for myocardial infarction.
Materials and methods
Ethics statement
Animal studies were approved by Soochow University Scientific and Animal Ethics Committee (approval no. 20120055) and were in compliance with Chinese national regulations on the use of experimental animals. Procedures for animal studies were performed in accordance with the Guide for the Care and Use of Laboratory Animals (revised in 1996) by the US National Institutes of Health (Bethesda, MD, USA).
Isolation and culture of Sca-1+CSCs
The present study used 6-week old, male C57/BL6 mice (weight, 15–20 g; n=20). All animals were purchased from the Laboratory Animal Center of Soochow University (Suzhou, China). They were maintained on standard diet and water with a 12-h light/dark cycle, room temperature and 50% humidity at the animal center of The First Affiliated Hospital of Soochow University.
CSCs were prepared following euthanasia with carbon dioxide. C57/BL6 mouse hearts were minced and digested with 0.1% collagenase B (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and 0.2% trypsin (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 3°C for 2 h. Cells were selected using a magnetic selection system (Miltenyi Biotec, Inc., Auburn, CA, USA). The purity of isolated cells was verified by flow cytometry. Isolated CSC were cultured in Dulbecco's modified Eagle's medium/nutrient mixture F12 (DMEM/F12) (Invitrogen; Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 10 ng/ml leukemia inhibitory factor (LIF; PeproTech, Inc., Rocky Hill, NJ, USA), 10 ng/ml cardiotroponin (PeproTech, Inc.), 10 ng/ml epidermal growth factor (EGF; PeproTech, Inc.) and a 1% solution of penicillin and streptomycin. The medium was replaced every three days.
Cell migration assay in vitro
A Transwell migration assay was used to measure cell migration in vitro. CSC were pre-washed with serum-free DMEM/F12 medium and 5×104 cells in 200 µl serum-free DMEM/F12 medium were seeded into the upper chamber of a 24 well Transwell plate (Merck KGaA). DMEM/F12 (600 µl) complete medium with different concentrations of bFGF, or bFGF plus the Akt inhibitor deguelin, was added to the lower chamber. Following incubation for 24 h, the upper surface of the membrane was scraped with a cotton swab to remove un-migrated cells. Cells migrating to the lower surface of the membrane were stained at room temperature for 20 min with 0.1% hexamethylpararosaniline. Each assay was performed in triplicate wells and repeated three times. The number of cells migrating to the lower surface of the membrane was counted in 10 random ×200 fields using an inverted microscope and the mean number of cells per field was used for statistical analysis. The Migration Index was calculated as Migration cell number (treated)/Migration cell number (untreated).
Myocardial infarction construction and cell transplantation
Myocardial infarction was simulated through surgical ligation of the left anterior descending (LAD) coronary artery with a 7/0 prolene suture (Ethicon, Inc., Somerville, NJ, USA) and successful ligation was verified through observation of a color change with the naked eye from red to white in the infarct area. 30 wild-type specific pathogen free C57/BL6 mice (8–10 weeks) were randomly assigned to three groups: CSC, bFGF-treated CSC (45 ng/ml) and bFGF-treated CSC + Akt inhibitor (5.0 mg/kg oral Akt inhibitor for 3 days prior to transplantation). For cell transplantation, animals received intra-myocardium injections of 1×106 cell solution immediately following LAD ligation. All animals were sacrificed one week following cell transplantation. A further 6 mice underwent LAD ligation without cell transplantation. bFGF protein expression and Sca-1+ cell aggregation was measured in these mice one week following simulated myocardial infarction.
Cell migration and maintenance assessment in vivo
Transplanted cells were labeled with the cell tracking dye CM-Dil (Invitrogen; Thermo Fisher Scientific, Inc.). Prior to cell transplantation, 5 µl CM-Dil was added to 1×106 cells suspended in 20 µl PBS and incubated at room temperature for 20 min. Successful labeling was confirmed by flow cytometry (BD FACSCalibur; BD Biosciences, Franklin Lakes, San Jose, CA, USA) and immunofluorescence microscopy. One week following transplantation, frozen tissue was obtained and sectioned into three continuous 2 µm sections. Cells maintained in the peri-infarct area and migrated to the infarct area were visualized as green and counted in 10 random ×200 fields using immunofluorescence microscopy for 10 sections. The mean number of cells per field was obtained for statistical analysis.
Western blot analysis
The harvested cells or minced tissues were lysed in a lysis buffer (Roche Diagnostics, Basel, Switzerland) at 37°C for 2 h. Protein concentrations were quantified using a bichoninic acid protein assay kit (Pierce; Thermo Fisher Scientific, Inc). 30 µg proteins were separated using 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a polyvinylidene difluoride (PVDF) membrane (EMD Millipore, Billerica, MA, USA). Following blocking the membrane with Tris-buffered saline + 0.1% Tween 20 (TBST) and 5% nonfat dried milk for 2 h at room temperature, the membrane was washed twice with TBST, then incubated with the primary antibody (Akt catalog no. ab 64148; 1:1,000; p-Akt catalog no. ab 38449; 1:1,000; bFGF catalog no. ab 168328; 1:1,500; CXCR-4 catalog no. ab1670; 1:1,000; SDF-1 catalog no. ab18919; 1:1,000; GAPDH catalog no. ab9485; 1:2,000; Abcam, Cambridge, UK) overnight at 4°C. Next, the membrane was washed with TBST, then incubated with the peroxidase-conjugated secondary antibody (catalog no. A21010 1:500; Wuhan Amyjet Scientific Co., Ltd., Wuhan, China). Bands were detected using a chemiluminescence western blot detection system (Pierce; Thermo Fisher Scientific, Inc.). The protein expression and phosphorylation levels were normalized to baseline expression and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) levels, respectively.
Immunohistochemistry
Hearts were fixed with 10% buffered formalin solution overnight, dehydrated in graded alcohols and embedded in paraffin. The tissue was subsequently cut into 2 µm thick sections, after blocking with Tris buffered saline Tween-20 (TBST) and 5% nonfat dried milk for 2 h, tissue sections were incubated at room temperature for 2 h with antibodies against bFGF (catalog no. ab168328; 1:500; Abcam). The Image-Pro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD, USA) was used to evaluate the positive expression ratio of bFGF.
Capillary density assessment
10% Formalin-fixed and paraffin-embedded tissue sections were sliced transversally into 2 µm sections and incubated with antibodies against von Willebrand factor (vWF) at room temperature for 2 h (catalog no. ab9378; 1:3,000; Abcam). Tissue was then washed twice with TBST and incubated with peroxidase-conjugated secondary antibodies (goat anti mouse; catalog no. A996702; 1:1,000; Wuhan Amyjet Scientific Co., Ltd.). Positive vessels were stained brown. The mean number of capillaries per field in the infarct myocardium was obtained by counting in 10 randomly selected ×200 fields for statistical analysis for 10 sections.
Statistical analysis
Data were expressed as the mean ± standard deviation and analyzed with SPSS 17.0 statistical software (SPSS, Inc., Chicago, IL, USA). Multiple comparisons were performed through one-way analysis of variance tests and Scheffe's post hoc tests when data were normally distributed, and through Kruskal-Wallis tests and Dunn's post hoc tests when data were not normally distributed. P<0.05 was considered to indicate a statistically significant difference.
Results
bFGF expression increases and Sca-1+ CSCs aggregate following myocardial infarction
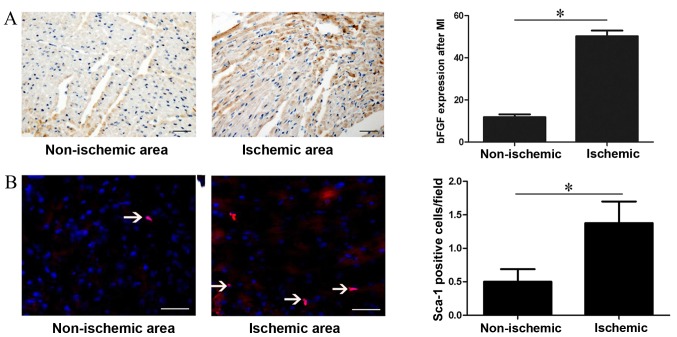
The expression of bFGF was analysed 1 week after myocardial infarction simulation. Immunohistochemical analysis revealed that bFGF expression was 50.3±2.61 in the ischemic area and 11.83±1.38 in the non-ischemic area (P=0.001; Fig. 1A). Immunofluorescence analysis demonstrated that regional ischemia following myocardial infarction resulted in increased recruitment of Sca-1+ CSCs in the ischemic area compared with the non-ischemic area (0.5±0.53 vs. 1.38±0.92, P=0.001; Fig. 1B).
Figure 1.
bFGF protein expression and Sca-1+ CSC recruitment following myocardial infarction. (A) Immunohistochemistry analysis of bFGF protein expression in different areas of the heart one week following myocardial infarction. bFGF was stained brown and nuclei were stained blue. (B) Immunofluorescent staining of Sca-1+ CSC in different areas of the heart one week after myocardial infarction. Consecutive 10 sections and 10 random ×200 fields per section were counted. *P<0.05, with comparisons indicated by lines. bFGF, basic fibroblast growth factor; CSC, cardiac stem cells. Bar=100 µm.
bFGF promotes Sca-1+ CSC migration in vitro in an Akt-dependent manner
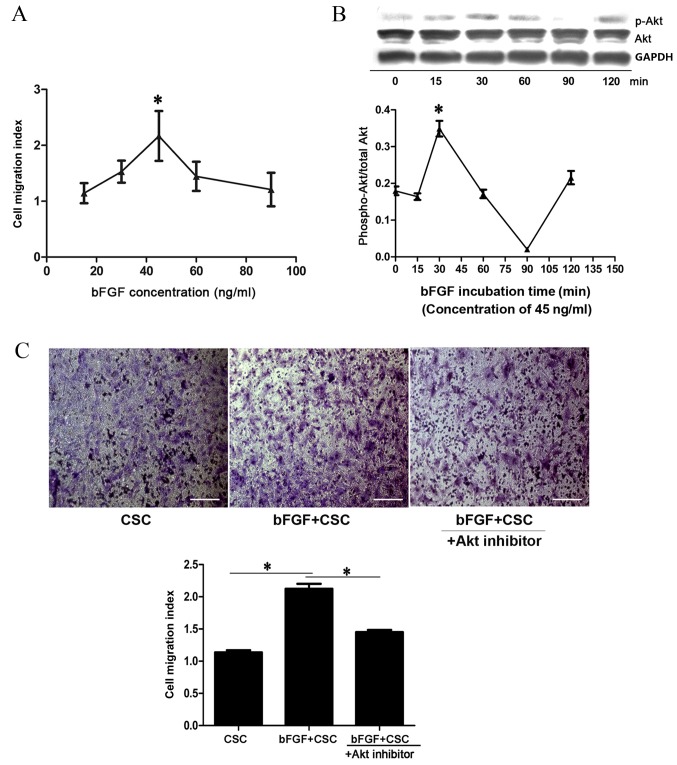
A Transwell assay was used to assess the effects of bFGF on Sca-1+ CSC migration in vitro. A concentration gradient of bFGF (15, 30, 45, 60 and 90 ng/ml) was used. The migration index of Sca-1+ CSCs increased with bFGF concentration and reached the highest level at 45 ng/ml (2.16±0.44; P=0.001 vs. 0 ng/ml; Fig. 2A), and decreased to baseline level at 90 ng/ml (1.21±0.30; Fig. 2A). To determine whether the PI3K/Akt pathway participates in bFGF-induced Sca-1+ CSC migration, cell samples treated with 45 ng/ml bFGF were collected at 0, 15, 30, 60, 90 and 120 min and the level of Akt activation was analysed by western blotting. The p-Akt/Akt ratio increased with time and reached the highest level at 30 min, suggesting a transient activation of p-Akt (0.34±0.02, P=0.001 vs. 0 min; Fig. 2B). In addition, an Akt inhibitor, deguelin, was used to determine whether bFGF dependent Sca-1+ CSC migration could be attenuated. The Sca-1+ CSC migration index was revealed to be 1.14±0.06 for CSCs, 2.12±0.13 for bFGF-treated CSCs (P=0.001 vs. CSC; Fig. 2C) and 1.45±0.06 for bFGF-treated CSCs + deguelin (P=0.004 vs. bFGF-treated CSCs; Fig. 2C). This suggests that the PI3K/Akt pathway is involved in CSC migration.
Figure 2.
bFGF promotes Sca-1+ CSC migration in vitro. (A) Cell migration index of Sca-1+ CSC treated with different bFGF concentrations. *P<0.05 vs. 15 ng/ml. (B) Western blot analysis of p-Akt and Akt at different time points following incubation with 45 ng/ml bFGF and quantification of p-Akt/Akt ratio. *P<0.05 vs. 0 min. (C) Cell migration assay and quantification. All migrated cells were stained purple with 0.1% hexamethylpararosaniline. Each assay was performed in triplicate wells and repeated three times. *P<0.05, with comparisons indicated by lines. bFGF, basic fibroblast growth factor; CSC, cardiac stem cells; p-, phosphorylated; Akt, protein kinase B.
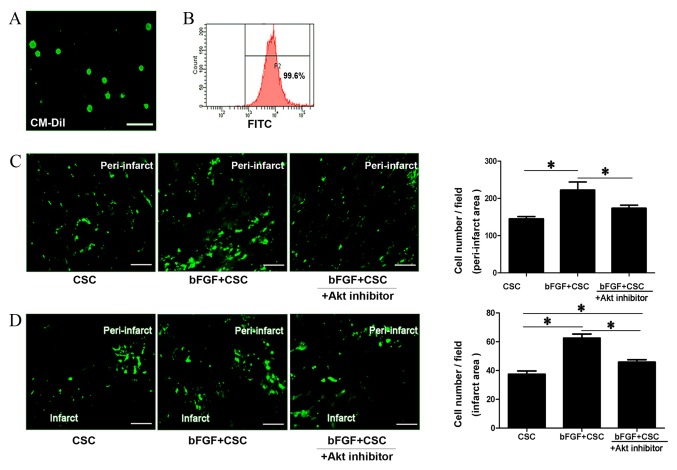
bFGF promoted cell maintenance and migration in vivo
Sca-1+ CSCs were labeled with CM-Dil cell tracking dye with a positive labeling rate of 99.6%, confirmed by flow cytometry and fluorescent microscopy (Fig. 3A and B). One week after cell transplantation, the viability of transplanted cells in the peri-infarct area and migrated cells in the infarct area were assessed via fluorescence microscopy. Sca-1+ CSCs demonstrated higher cell maintenance rates in the peri-infarct area when treated with bFGF (control CSCs, 166.4±20.45 cells/field; bFGF-treated CSCs, 222.4±47.95 cells/field; P=0.001; Fig. 3C) and the Akt inhibitor deguelin reduced this effect (bFGF-treated CSCs + deguelin, 173.6±18.39 cells/field; P=0.001 vs. bFGF-treated CSC; Fig. 3C). Cell number in the infarct area, reflecting the migratory ability of the transplanted cells, was also examined. Sca-1+ CSCs demonstrated improved migratory ability when treated with bFGF, with increased numbers of cells migrating into the infarct myocardium (control CSCs, 37.43±5.95 cells/field; bFGF-treated CSCs, 62.58±7.34 cells/field; P=0.001; Fig. 3D). This effect was partially blocked by the Akt inhibitor deguelin (bFGF-treated CSC + deguelin 45.85±4.33 cells/field; P=0.001 vs. control CSCs, P=0.001 vs bFGF-treated CSCs; Fig. 3D). This suggests that bFGF delivery promotes CSC maintenance in the peri-infarct area and cell migration to the infarct area, and these effects were blocked by an Akt inhibitor.
Figure 3.
bFGF promotes Sca-1+ CSC maintenance and migration in vivo. (A) CM-Dil cell tracking dye labeling on Sca-1+ CSCs. Labeled cells were observed as green under fluorescence microscopy. (B) Flow cytometry confirmation that the positive labeling rate reached 99.6%. (C) Maintenance of transplanted CSCs (green) in the peri-infarct area one week after cell transplantation, and quantitation. (D) Maintenance of transplanted CSC in the infarct area one week following cell transplantation, reflecting the migratory ability of transplanted cells, and quantitation. Consecutive 10 sections and 10 random ×200 fields per section were counted. *P<0.05, with comparisons indicated by lines. bFGF, basic fibroblast growth factor; CSC, cardiac stem cells; Akt, protein kinase B.
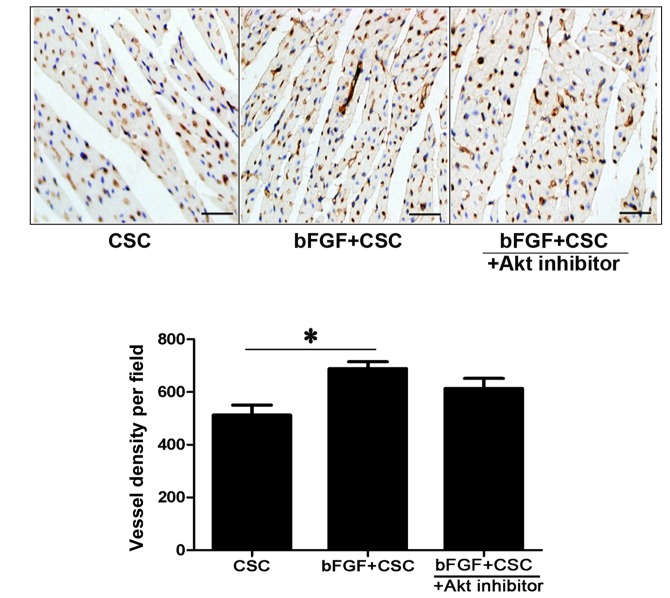
bFGF-treated CSC transplantation increases capillary density in the infarcted myocardium
vWF immunohistochemistry was used to measure capillary density in the infarcted myocardium. The mean number of micro-vessels per field in the peri-infarct myocardium was increased in the bFGF-treated CSC group (control CSC 512.5±75.76 vessels/field; bFGF-treated CSC 689.25±51.73 vessels/field; P=0.001; Fig. 4). This effect was partially attenuated by the Akt inhibitor deguelin (bFGF-treated CSC + deguelin 613.5±76.44 vessels/field; Fig. 4) but no significant difference was observed between the bFGF-treated CSC group and the bFGF-treated CSC + deguelin group.
Figure 4.
bFGF increases angiogenesis in vivo. Measurement of capillary density by vWF immunohistochemistry in the peri-infarct myocardium one week after cell transplantation, and quantitation. Capillaries are stained dark brown and nuclei are stained blue. Consecutive 10 sections and 10 random ×200 fields per section were counted. *P<0.05, with comparisons indicated by lines. bFGF, basic fibroblast growth factor; CSC, cardiac stem cells; Akt, protein kinase B.
Upregulation of bFGF, CXCR-4 and SDF-1 is Akt dependent
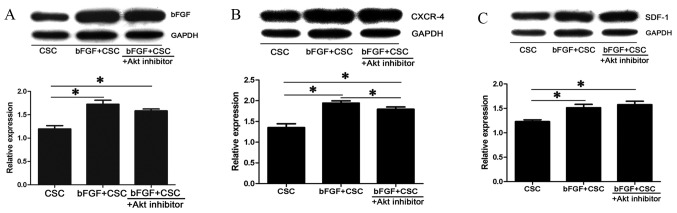
Western blot analysis was performed to evaluate protein expression of bFGF, CXCR-4 and SDF-1. An up-regulation of bFGF (1.72±0.14 fold; P=0.001; Fig. 5A), CXCR-4 (1.94±0.1 fold; P=0.001; Fig. 5B) and SDF-1 (1.51±0.12 fold; P=0.001; Fig. 5C) was observed in the bFGF-treated CSC group compared with the control CSC group following cell transplantation. This upregulation of bFGF and CXCR-4 was partially attenuated by exogenous Akt inhibitor, with smaller increases in protein expression of bFGF (1.58±0.07 fold; P=0.001; Fig. 5A), CXCR-4 (1.79±0.09 fold; P=0.001 vs. CSC and P=0.001 vs. bFGF-treated CSC; Fig. 5B) in the bFGF-treated CSC + deguelin group compared with the control CSC group.
Figure 5.
Activation of CXCR-4 and SDF-1 following bFGF treatment. Protein expression levels, with quantitation relative to GAPDH, of (A) bFGF, (B) CXCR-4 and (C) SDF-1 in the peri-infarct myocardium one week following cell transplantation, measured by western blot analysis. Each assay was repeated three times. *P<0.05, with comparisons indicated by lines. CXCR-4, C-X-C chemokine receptor type 4; SDF-1, stromal cell derived factor 1; bFGF, basic fibroblast growth factor; CSC, cardiac stem cells; Akt, protein kinase B; GADPH, glyceraldehyde 3-phosphate dehydrogenase.
Discussion
The present study demonstrated that bFGF treatment promoted the migratory ability of cultured CSCs. This effect was observed in vitro and in vivo, with increased CSC migration into the infarcted myocardium observed in the bFGF-treated group. It was hypothesized that this effect may have been mediated through the PI3K/Akt pathway, with specific blocking of this pathway attenuating the effect of bFGF on CSC migration.
The present study demonstrated that expression of bFGF was elevated following myocardial infarction in both the ischemic and peri-ischemic area. Immunofluorescence staining revealed increased numbers of Sca-1+ CSCs in the peri-ischemic area, suggesting links between Sca-1+ CSC recruitment and bFGF concentration. Ischemia and hypoxia can stimulate the release of bFGF from the intracellular and extra-cellular matrices, and it binds to specific fibroblast growth factor receptors on the cell membrane (17). bFGF is known to have potent angiogenic abilities, and is able to protect cardiomyocytes from ischemic/reperfusion injury by promoting capillary formation and increasing micro-vessel density, which ameliorates the local blood supply (27,28). In a rabbit myocardial infarction model, intra-myocardial injection of bFGF promoted vascular endothelium recovery and the maintenance of cardiac function (29). The present study revealed that bFGF-treated CSC transplantation led to improved angiogenesis and increased vessel density. It was hypothesized that angiogenesis increased the efficiency of stem cell transplantation by facilitating cell survival and migration due to an increased supply of blood and oxygen. This was in accordance with the observation that there was increased stem cell maintenance in the implanted area (30).
The migration of stem cells primarily occurs through chemotaxis. Many signaling pathways, such as PI3K/Akt and the SDF-1/CXCR-4 axis are involved in the mobilization, migration, homing, and implantation of stem cells (11–13). Myocardial infarction is an inflammatory response resulting from ischemia and hypoxia, and is accompanied with augmented release of cytokines such as VEGF, bFGF and HIF in the local area. These are all important chemotaxis factors involved in cell migration (31–33). Under the control of these cytokines, SDF-1 recruits bone marrow and peripheral stem cells into the ischemic and peri-ischemic area, where the concentration is greatest (34). The present study observed the up-regulation of SDF-1 and CXCR-4 in the bFGF-treated group, coinciding with the improved migratory ability in this group. When the PI3K/Akt pathway was blocked by an inhibitor, the migration of CSCs was inhibited and the expression of SDF-1 and CXCR-4 also decreased. This suggests that the PI3K/Akt pathway may partially modulate the SDF-1/CXCR-4 axis in the process of cell migration.
To conclude, the present study demonstrated that bFGF promoted the migration of CSCs, both in vitro and in vivo. This effect was partially mediated through the PI3K/Akt pathway. Transplantation of bFGF-treated CSCs in a murine myocardial infarction model increased CSC maintenance and migration, as well as local angiogenesis. This pro-migratory effect of bFGF demonstrates that its importance to myocardial regeneration extends beyond its traditional angiogeneic ability. This understanding, if incorporated into CSC therapy, may improve its efficiency when treating myocardial infarct. The limitation of this study was that the CSCs were transplanted through myocardium injection. A more convenient method could have been intravenous injection but the best method for cell transplantation requires further study.
Acknowledgements
The present study was supported by The Youth Science and Technology of Suzhou Science and Education Project (grant no. KJXW2013004), The Youth Science Foundation of Jiangsu Province, China (grant no. BK20140296) and The Science Foundation for Youth Teacher of Soochow University (grant no. SDY2013A29).
References
- 1.Anderson KM. Discharge clinical characteristics and 60-day readmission in patients hospitalized with heart failure. J Cardiovasc Nurs. 2014;29:232–241. doi: 10.1097/JCN.0b013e31828f0d25. [DOI] [PubMed] [Google Scholar]
- 2.Noseda M, Abreu-Paiva M, Schneider MD. The Quest for the adult cardiac stem cell. Circ J. 2015;79:1422–1430. doi: 10.1253/circj.CJ-15-0557. [DOI] [PubMed] [Google Scholar]
- 3.Mosna F, Annunziato F, Pizzolo G, Krampera M. Cell therapy for cardiac regeneration after myocardial infarct: Which cell is the best? Cardiovasc Hematol Agents Med Chem. 2010;8:227–243. doi: 10.2174/187152510792481216. [DOI] [PubMed] [Google Scholar]
- 4.Nadal-Ginard B, Ellison GM, Torella D. The cardiac stem cell compartment is indispensable for myocardial cell homeostasis, repair and regeneration in the adult. Stem Cell Res. 2014;13:615–630. doi: 10.1016/j.scr.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Barile L, Messina E, Giacomello A, Marbán E. Endogenous cardiac stem cells. Prog Cardiovasc Dis. 2007;50:31–48. doi: 10.1016/j.pcad.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, et al. Human cardiac stem cells; Proc Natl Acad Sci USA; 2007; pp. 14068–14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forte G, Minieri M, Cossa P, Antenucci D, Sala M, Gnocchi V, Fiaccavento R, Carotenuto F, De Vito P, Baldini PM, et al. Hepatocyte growth factor effects on mesenchymal stem cells: Proliferation, migration, and differentiation. Stem Cells. 2006;24:23–33. doi: 10.1634/stemcells.2004-0176. [DOI] [PubMed] [Google Scholar]
- 9.Tang J, Wang J, Kong X, Yang J, Guo L, Zheng F, Zhang L, Huang Y, Wan Y. Vascular endothelial growth factor promotes cardiac stem cell migration via the PI3K/Akt pathway. Exp Cell Res. 2009;315:3521–3531. doi: 10.1016/j.yexcr.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 10.Tang JM, Wang JN, Zhang L, Zheng F, Yang JY, Kong X, Guo LY, Chen L, Huang YZ, Wan Y, Chen SY. VEGF/SDF-1 promotes cardiac stem cell mobilization and myocardial repair in the infarcted heart. Cardiovasc Res. 2011;91:402–411. doi: 10.1093/cvr/cvr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Unzek S, Zhang M, Mal N, Mills WR, Laurita KR, Penn MS. SDF-1 recruits cardiac stem cell-like cells that depolarize in vivo. Cell Transplant. 2007;16:879–886. doi: 10.3727/096368907783338271. [DOI] [PubMed] [Google Scholar]
- 12.Madonna R, Rokosh G, De Caterina R, Bolli R. Hepatocyte growth factor/Met gene transfer in cardiac stem cells-potential for cardiac repair. Basic Res Cardiol. 2010;105:443–452. doi: 10.1007/s00395-010-0102-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urbanek K, Rota M, Cascapera S, Bearzi C, Nascimbene A, De Angelis A, Hosoda T, Chimenti S, Baker M, Limana F, et al. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res. 2005;97:663–673. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]
- 14.Takehara N, Tsutsumi Y, Tateishi K, Ogata T, Tanaka H, Ueyama T, Takahashi T, Takamatsu T, Fukushima M, Komeda M, et al. Controlled delivery of basic fibroblast growth factor promotes human cardiosphere-derived cell engraftment to enhance cardiac repair for chronic myocardial infarction. J Am Coll Cardiol. 2008;52:1858–1865. doi: 10.1016/j.jacc.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 15.Virag JA, Rolle ML, Reece J, Hardouin S, Feigl EO, Murry CE. Fibroblast growth factor-2 regulates myocardial infarct repair: Effects on cell proliferation, scar contraction, and ventricular function. Am J Pathol. 2007;171:1431–1440. doi: 10.2353/ajpath.2007.070003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tabata Y, Ikada Y. Vascularization effect of basic fibroblast growth factor released from gelatin hydrogels with different biodegradabilities. Biomaterials. 1999;20:2169–2175. doi: 10.1016/S0142-9612(99)00121-0. [DOI] [PubMed] [Google Scholar]
- 17.Boodhwani M, Voisine P, Ruel M, Sodha NR, Feng J, Xu SH, Bianchi C, Sellke FW. Comparison of vascular endothelial growth factor and fibroblast growth factor-2 in a swine model of endothelial dysfunction. Eur J Cardiothorac Surg. 2008;33:645–650. doi: 10.1016/j.ejcts.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cuevas P, Barrios V, Giménez-Gallego G, Martinez-Coso V, Cuevas B, Benavides J, Garcia-Segovia J, Asin-Cardiel E. Serum levels of basic fibroblast growth factor in acute myocardial infarction. Eur J Med Res. 1997;2:282–284. [PubMed] [Google Scholar]
- 19.Fujita M, Ikemoto M, Kishishita M, Otani H, Nohara R, Tanaka T, Tamaki S, Yamazato A, Sasayama S. Elevated basic fibroblast growth factor in pericardial fluid of patients with unstable angina. Circulation. 1996;94:610–613. doi: 10.1161/01.CIR.94.4.610. [DOI] [PubMed] [Google Scholar]
- 20.Anversa P, Rota M, Urbanek K, Hosoda T, Sonnenblick EH, Leri A, Kajstura J, Bolli R. Myocardial aging-a stem cell problem. Basic Res Cardiol. 2005;100:482–493. doi: 10.1007/s00395-005-0554-3. [DOI] [PubMed] [Google Scholar]
- 21.Linke A, Müller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, Castaldo C, Cascapera S, Böhm M, Quaini F, et al. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function; Proc Natl Acad Sci USA; 2005; pp. 8966–8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Urbanek K, Torella D, Sheikh F, De Angelis A, Nurzynska D, Silvestri F, Beltrami CA, Bussani R, Beltrami AP, Quaini F, et al. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure; Proc Natl Acad Sci USA; 2005; pp. 8692–8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith RR, Barile L, Messina E, Marbán E. Stem cells in the heart: What's the buzz all about?––Part 1: Preclinical considerations. Heart Rhythm. 2008;5:749–757. doi: 10.1016/j.hrthm.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Hu Q, Nakamura Y, Lee J, Zhang G, From AH, Zhang J. The role of the sca-1+/CD31- cardiac progenitor cell population in postinfarction left ventricular remodeling. Stem Cells. 2006;24:1779–1788. doi: 10.1634/stemcells.2005-0386. [DOI] [PubMed] [Google Scholar]
- 25.Müller-Ehmsen J, Krausgrill B, Burst V, Schenk K, Neisen UC, Fries JW, Fleischmann BK, Hescheler J, Schwinger RHG. Effective engraftment but poor mid-term persistence of mononuclear and mesenchymal bone marrow cells in acute and chronic rat myocardial infarction. J Mol Cell Cardiol. 2006;41:876–884. doi: 10.1016/j.yjmcc.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 26.Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: Graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33:907–921. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 27.Iwakura A, Fujita M, Kataoka K, Tambara K, Sakakibara Y, Komeda M, Tabata Y. Intramyocardial sustained delivery of basic fibroblast growth factor improves angiogenesis and ventricular function in a rat infarct model. Heart Vessels. 2000;18:93–99. doi: 10.1007/s10380-002-0686-5. [DOI] [PubMed] [Google Scholar]
- 28.Kawasuji M, Nagamine H, Ikeda M, Sakakibara N, Takemura H, Fujii S, Watanabe Y. Therapeutic angiogenesis with intramyocardial administration of basic fibroblast growth factor. Ann Thorac Surg. 2000;69:1155–1161. doi: 10.1016/S0003-4975(99)01557-X. [DOI] [PubMed] [Google Scholar]
- 29.Bougioukas I, Didilis V, Ypsilantis P, Giatromanolaki A, Sivridis E, Lialiaris T, Mikroulis D, Simopoulos C, Bougioukas G. Intramyocardial injection of low-dose basic fibroblast growth factor or vascular endothelial growth factor induces angiogenesis in the infarcted rabbit myocardium. Cardiovasc Pathol. 2007;16:63–68. doi: 10.1016/j.carpath.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101:660–667. doi: 10.1161/01.CIR.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nervi B, Link DC, DiPersio JF. Cytokines and hematopoietic stem cell mobilization. J Cell Biochem. 2006;99:690–705. doi: 10.1002/jcb.21043. [DOI] [PubMed] [Google Scholar]
- 32.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 34.Kucia M, Jankowski K, Reca R, Wysoczynski M, Bandura L, Allendorf DJ, Zhang J, Ratajczak J, Ratajczak MZ. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004;35:233–245. doi: 10.1023/B:HIJO.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]