Abstract
Introduction
US obstetrician/gynecologists (ob-gyns) play a critical role as vaccinators of pregnant women. However, little is known about their current immunization practices. Thus, study objectives were to determine: 1) practices related to assessment of vaccination status and vaccine delivery for pregnant patients; 2) barriers to stocking and administering vaccines; and 3) factors associated with administering both influenza and tetanus, diphtheria, and acellular pertussis (Tdap) vaccines.
Methods
An e-mail and mail survey among a national sample of ob-gyns conducted July-October 2015 (analysis August 2016-August 2017).
Results
The response rate was 73.2% (353/482). Among ob-gyn’s caring for pregnant women (n=324), vaccination status was most commonly assessed for influenza (97%), Tdap (92%), and measles, mumps, and rubella (MMR) vaccines (88%). Vaccines most commonly administered included influenza (85%) and Tdap (76%). Few respondents reported administering other vaccines to pregnant patients. More physicians reported using standing orders for influenza (66%) than Tdap (39%). Other evidence-based strategies for increasing vaccine uptake were less frequently used (electronic decision support, 42%; immunization information system (IIS) to record (13%) or assess vaccination status (11%); reminder/recall, 7%). Barriers most commonly reported were provider financial barriers; provider attitudinal barriers were rare. Providers who administered both influenza and Tdap vaccines were more likely to be female, perceive fewer financial and practice barriers and less likely to be in private practice and perceive more patient barriers.
Conclusion
While most ob-gyns administer some vaccines to pregnant women, the focus remains on influenza and Tdap. Financial barriers and infrequent use of evidence-based strategies for increasing vaccination uptake may be hindering delivery of a broader complement of adult vaccines in ob-gyn offices.
INTRODUCTION
Pregnant women are at increased risk for severe disease from influenza,1–5 and their newborns are at increased risk of morbidity and mortality from both influenza6–8 and pertussis.9,10 Influenza and pertussis vaccination are therefore now routinely recommended for all pregnant women,11,12 with influenza vaccination recommendations in place since 2004. The initial recommendation for tetanus, diphtheria, and acellular pertussis (Tdap) vaccination in 2011 was for pregnant women with no history of prior Tdap, but, in 2012, was extended to all pregnant women during each pregnancy. Both vaccines are safe and effective.13–27
However, despite the benefits of these vaccines for pregnant women and their newborns, uptake remains low: according to the most recent national data, only 50% of pregnant women received influenza vaccine before or during pregnancy and only 41% of pregnant women received Tdap during pregnancy.28–31 While some of the barriers to increased uptake are related to patient concerns about vaccine safety in pregnancy,32 there are also provider barriers. In a recent national survey, a substantial proportion of pregnant women reported receiving neither a recommendation nor an offer for an influenza vaccine from a provider. These women were far less likely to receive an influenza vaccine (20%) than those who received both a recommendation and an offer (65%).28
There are other vaccines recommended for pregnant women in certain circumstances, such as hepatitis A and B vaccines, pneumococcal vaccines, and meningococcal vaccines.33 Pregnancy represents an opportunity for adult vaccination given the high number of contacts with the obstetrician/gynecologist’s (ob-gyn) office during a routine pregnancy. Little is known about ob-gyn current practices regarding use of all indicated vaccines in pregnancy, as there has been no recent national assessment. This study sought to address this gap in the literature by assessing among a national sample of ob-gyns: 1) current practices related to assessment of vaccination status and vaccine delivery for pregnant patients; 2) barriers to stocking and administering vaccines in ob-gyn practices; and 3) factors associated with administering both influenza and Tdap vaccines to pregnant patients.
METHODS
Between 7/2015 and 10/2015, an Internet and mail survey was administered to a national network of ob-gyns representative of American College of Obstetricians and Gynecologists (ACOG) membership. The IRB at the University of Colorado Denver deemed this study exempt research not requiring informed consent.
Study Population
The Vaccine Policy Collaborative Initiative conducted this study.34 The Initiative is a program designed collaboratively with CDC to perform rapid turnaround surveys assessing physician practices and attitudes about vaccine-related issues. A national network of ob-gyns was developed for this program by recruiting from ACOG. Quota sampling was conducted to ensure that network physicians were similar to ACOG membership with respect to region, urban versus rural location, and practice setting. In prior work, survey responses from network physicians compared to those of physicians randomly sampled from American Medical Association physician databases were similar in regard to demographic characteristics, practice attributes, and attitudes about a range of vaccination issues.34 Physicians who reported that they only cared for non-pregnant patients were excluded from this study.
Survey Design
The survey was jointly developed with CDC with input from experts in vaccination and obstetrics and gynecology. Survey questions followed formats previously used in published surveys.35–37 The survey was pre-tested with a panel of 6 ob-gyns and then piloted among 38 ob-gyns from different regions of the country. Questions regarding assessing and administering vaccines and use of evidence-based practices were asked using a series of yes/no questions. Questions regarding frequency of a given practice were assessed using a 4-point Likert scale (never/rarely, sometimes, often, always). Barriers questions also used a 4-point Likert scale from ‘not a barrier’ to ‘major barrier.’ Other responses to information questions were either yes/no, answers that were not mutually exclusive, or selections from a list of possible options.
Survey Administration
Physicians were surveyed via Internet or mail according to their preference. A Web-based program (Verint®, Melville, New York, www.verint.com) was used to administer Internet surveys, and we sent mail surveys by U.S. Postal Service. The Internet group was sent an initial e-mail with up to 8 e-mail reminders, and the mail group was sent an initial mailing and up to 2 reminders. Internet survey non-respondents were sent a cross-over mail-based survey in case of problems with e-mail correspondence. The mail protocol was patterned on Dillman’s tailored design method.38
Statistical Analysis
Internet and mail surveys were pooled for analyses because other studies have found that physician attitudes are similar with either method.39 Respondents were compared with non-respondents on all available characteristics using Wilcoxon and chi-square analyses.
Physicians who responded ‘yes’ to the query “Do you give the vaccine in your practice?” for both influenza and Tdap vaccines were compared to those who responded ‘no’ for either or both vaccines. Independent variables included sex, age, practice setting, practice location, practice region, and perceived barriers. Perceived barriers were evaluated and grouped using a Principal Component Analysis with varimax rotation. Factors were retained if their eigenvalue was ≥1. A cut-off of p<0.25 was used for inclusion of independent variables in the model. The multivariable model used a backwards elimination procedure in which the least significant predictor in the model was eliminated sequentially. At each step, estimates were checked to make sure other variables were not affected by dropping the least significant variable. This resulted in retention of only those factors that were significant at p<0.05 in the final model. Analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, North Carolina) August 2016 to August 2017.
RESULTS
The response rate was 73.2% (353/482). Respondents were more likely than non-respondents to be female, work in a hospital-associated clinic, and have a higher median and mean number of providers in their office (Table 1). Overall, 90% reported stocking and administering at least one vaccine.
Table 1.
Respondent and Non-Respondent and Other Practice Characteristics of US Obstetrician-Gynecologists Surveyed, 2015 (n=482)
| Characteristic | OB-GYN | ||
|---|---|---|---|
| Respondents (n=353) | Non-Respondents (n=129) | P-Valuea | |
| Age in years, mean (SD) | 48.9 (10.8) | 49.2 (10.6) | 0.86 |
| Male, % | 29.9 | 42.2 | 0.01 |
|
Region, % Midwest |
21.3 | 18.6 | |
| Northeast | 19.8 | 26.4 | |
| South | 36.0 | 36.4 | |
| West | 23.0 | 18.6 | 0.39 |
|
Location of Practice, % Urban |
55.5 | 57.4 | |
| Suburban | 41.6 | 41.1 | |
| Rural | 2.8 | 1.6 | 0.71 |
|
Setting, % Private practice |
64.9 | 76.6 | |
| Hospital/clinic | 27.3 | 15.6 | |
| HMO | 7.8 | 7.8 | 0.03 |
|
Number of Sites in Multi-site System, % 2–3 |
17.6 | NA | |
| 4–6 | 22.9 | NA | |
| 7–9 | 15.4 | NA | |
| ≥10 | 44.1 | NA | NA |
Wilcoxon test
Abbreviations: OB-GYN, obstetrician-gynecologist; HMO, health maintenance organization.
Note: Boldface indicates statistical significance (p<0.05)
Assessment of Vaccination Status
Ob-gyn physicians were the provider most frequently reported as primarily responsible for assessing vaccination status (72%), followed by medical assistants (MA)/licensed professional nurses (LPN) (12%), registered nurses (9%), and advanced care providers (nurse midwife, nurse practitioner, physician assistant) (6%). Two percent reported no routine assessment. Respondents reported multiple methods for assessing vaccination status, including checking their own medical records (87%), ob-gyn (85%) and staff (66%) asking patients verbally, physician reviewing outside records (61%), asking in a standard questionnaire (60%), staff reviewing outside records (32%), and using an immunization information system (IIS) (11%). Respondents reported routinely assessing vaccination status (other than influenza) most often at initial visits (93%), followed by third trimester (64%), first trimester (45%), and second trimester (40%), with 16% reporting they routinely assess vaccination status at every visit.
Respondents most commonly reported assessing vaccination status of pregnant women for influenza (97%) and Tdap (92%) vaccines (Figure 1). Other vaccines frequently assessed during pregnancy included MMR (88%), hepatitis B (77%), and varicella (50%). Ninety-eight percent of physicians reported assessing serology for rubella and 44% for varicella.
Figure 1. Assessment of Vaccination Status and Vaccines Administered to Pregnant Patients by US Obstetrician-Gynecologists, 2015 (n=324).
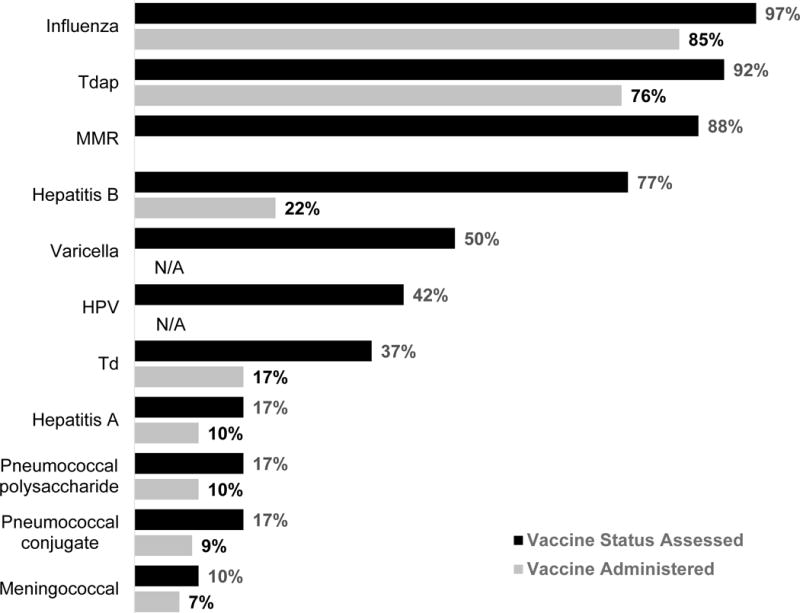
Tdap, tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine; MMR, measles, mumps and rubella vaccine; HPV, human papillomavirus vaccine; Td=tetanus and diphtheria toxoid vaccine; assessment for pneumococcal vaccination status was generic and not specific to either vaccine.
Few respondents reported recording information regarding vaccines received either in the office (13%) or outside the office (10%) in an IIS. For vaccines received outside the office, the most common method of recording information was in a progress note in an electronic medical or electronic health record (EMR/EHR) (69%), followed by a summary sheet in the EMR/EHR (61%), a progress note in a paper-based record (19%), and a summary sheet in a paper-based record (11%). Ten percent of respondents reported not recording this information anywhere.
Vaccine Administration
Vaccines most commonly administered in the office to pregnant patients included influenza (85%) and Tdap (76%) (Figure 1). Vaccines less commonly administered, in descending order, included hepatitis B (22%), tetanus and diphtheria (Td) (17%), hepatitis A (10%), pneumococcal polysaccharide (10%), pneumococcal conjugate (9%), and meningococcal conjugate (7%) vaccines. In cases where a patient was identified as eligible for a vaccine that the practice did not stock, the majority of physicians always or often (56%) referred them to their primary care provider to receive the vaccine, with fewer referring to a public health department (32%) or pharmacy (25%).
Use of Evidence-based Strategies and Vaccination-Related Resources
Most respondents (66%) reported using standing orders for influenza vaccine although only 39% reported doing so for Tdap. The only other strategy commonly used was electronic clinical decision support systems for determining vaccination need (42%) with fewer reporting use of paper-based clinical decision support (16%) or reminder/recall (7%). The most commonly used resources were primarily CDC materials such as Tdap materials on maternal immunization, printed immunization schedules, and the CDC immunization website, although relatively high proportions of physicians reported rarely or never using any of the materials. Few physicians frequently used ACOG-developed materials. CDC and ACOG scheduling ‘apps’ were the least used resources.
Barriers to Stocking and Administering Vaccines
The most commonly cited barriers to stocking and administering vaccines were financial (Figure 2). Fifty-three percent of respondents reported lack of adequate reimbursement for vaccine purchase and 45% reported lack of adequate reimbursement for vaccine administration as major or moderate barriers. There were also logistical barriers such as other preventive services taking precedence (47% major or moderate barrier) and the burden of ordering and tracking (45% major or moderate barrier) or storing (41% major or moderate barrier) vaccines. Patient attitudinal barriers were also commonly reported, with 52% reporting patients refusing vaccines because of safety concerns as a major or moderate barrier and 40% reporting patients refusing because they don’t believe they are at risk for a vaccine-preventable disease. In contrast, attitudinal barriers regarding vaccines among ob-gyns themselves were quite rare, with few or no respondents reporting as major or moderate barriers their concerns about vaccine effectiveness or safety, or personal concerns that their patients were not at risk of serious disease from vaccine-preventable illness.
Figure 2. Barriers to Stocking and Administering Vaccines among US Obstetrician-Gynecologists, 2015 (n=324).
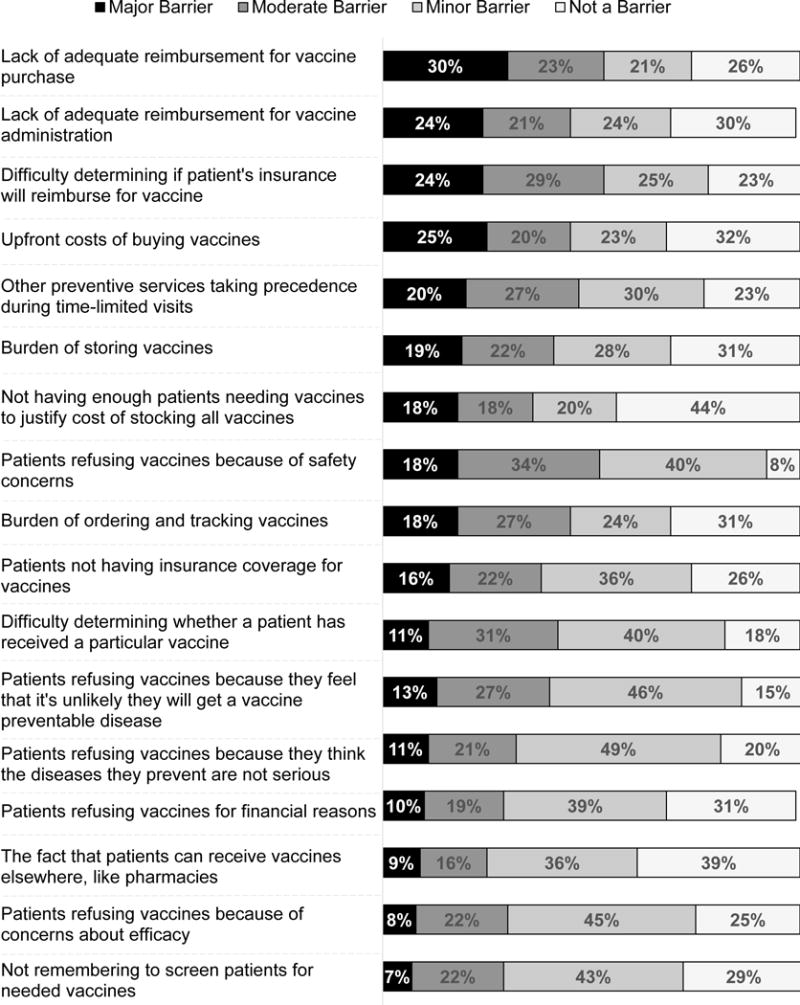
Some percentages do not add up to 100% due to rounding
Factors Associated with Giving Both Influenza and Tdap Vaccines
In bivariate analysis, female gender, decreasing age, non-private practice setting, not being in the South, lower scores on financial and practice barriers, and higher scores on patient barriers were associated with giving both Tdap and influenza vaccines (Table 2). In multivariable analysis, however, the age effect and South region fell out of the model, so that after adjustment, factors associated with giving both influenza and Tdap vaccines included female gender (prevalence ratio [PR], 0.78 male referent to female, 95% CI, 0.66–0.93), not being in private practice (1.23 [1.08–1.39]), lower perceived financial (0.86 [0.77–0.96]) and practice (0.87 [0.78–0.97]) barriers, and higher perceived patient barriers (1.16 [1.07–1.26]).
Table 2.
Factors Associated with Giving Both Tdap and Influenza Vaccines Among US Obstetricians-Gynecologists, 2015 (n+294)a
| Variable | Does not give both Tdap and influenza vaccines n=78 (27%) % |
Gives both Tdap and influenza vaccines n=216 (73%) % |
Bivariate PR (95% CI) | Multivariable PR (95% CI) |
|---|---|---|---|---|
|
| ||||
| Gender** | ||||
| Male | 49 | 25 | 0.73 (0.60–0.88) | 0.78 (0.66–0.93) |
| Female | 51 | 75 | Ref. | Ref. |
|
| ||||
| Mean (sd) age in years | 52.7 (11.0) | 46.7 (10.6) | 0.93 (0.90–0.96) per 5 years | |
|
| ||||
| Setting** | ||||
| Private practice | 86 | 54 | Ref. | Ref. |
| Other (Hospital/clinic, HMO, Public Health, University) | 14 | 46 | 1.43 (1.25–1.62) | 1.23 (1.08–1.39) |
|
| ||||
| Region | ||||
| Midwest | 15 | 24 | 1.28 (1.06–1.54) | |
| Northeast | 19 | 18 | 1.14 (0.91–1.42) | |
| South | 49 | 31 | Ref. | |
| West | 17 | 28 | 1.30 (1.08–1.55) | |
|
| ||||
| Mean (sd) Factor 1* Financial Barriers (per 1 point)b | 1.9 (0.8) | 1.1 (0.8) | 0.82 (0.77–0.88) | 0.86 (0.77–0.96) |
|
| ||||
| Mean (sd) Factor 2** Patient Attitudinal Barriers (per 1 point) | 1.2 (0.7) | 1.4 (0.7) | 1.11 (1.03–1.21) | 1.16 (1.07–1.26) |
|
| ||||
| Mean (sd) Factor 3* Practice Barriers (per 1 point) | 1.7 (0.9) | 1.0 (0.8) | 0.82 (0.76–0.88) | 0.87 (0.78–0.97) |
|
| ||||
| Mean (sd) Factor 4 Visit-level Barriers (per 1 point) | 1.3 (0.8) | 1.3 (0.7) | 0.99 (0.90–1.08) | |
Abbreviations: Tdap, tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine; PR, prevalence ratio; CI, confidence interval; ref., reference; sd, standard deviation; HMO, health maintenance organization.
n of 294 includes physicians providing care to pregnant patients with non-missing outcomes
Cronbach’s alpha for factors: 1=0.89; 2=0.87; 3=0.80; 4=0.68
Note: Boldface indicates statistical significance (*p<0.05, **p<0.01)
DISCUSSION
In this national survey of US ob-gyns, almost all report assessing vaccination status for influenza and Tdap vaccine among their pregnant patients, and most report stocking and administering these vaccines in their practice as well. This study represents the first data regarding the use of Tdap vaccine by ob-gyns since the changes in recommendations that occurred in 2011 and 2012. Three-quarters of ob-gyns now report administering the vaccine. However, relatively fewer ob-gyns assess vaccination status for other vaccines potentially recommended during a pregnancy, and even fewer administer other vaccines that pregnant women may need in certain circumstances, such as hepatitis A or B vaccines. Also, few obstetricians participate in an IIS.
This study offers a current benchmark for obstetricians’ practices regarding assessment and administration of vaccines to pregnant women. The last national surveys on this topic were performed in 200740 and 2009,41 although the latter had a very low response rate. In the 2007 study, Power et al reported that 79% stock and administer at least some vaccines, and among those, 61% of providers stocked and administered influenza vaccine to pregnant women, which translates to about 48% of obstetricians administering influenza vaccine to pregnant women. The 2009 study, performed during the H1N1 pandemic season, reported 71% administered influenza vaccine, although that survey was more prone to response bias given a response rate of 15%. This study suggests that there has been substantial progress, with 85% of ob-gyns who care for pregnant women now administering influenza vaccine to their pregnant patients. Stocking the vaccine matters, as previous work has shown that women who received both a recommendation and an offer of vaccination were about twice as likely to receive influenza vaccine as those who received a recommendation alone.28 It is noteworthy that while approximately half of providers in 2007 stocked and administered influenza vaccine to pregnant women, vaccination coverage for influenza vaccine in the 2007–2008 season among pregnant women was only 27%42 compared to 50% in the 2014–2015 season. There is no evidence that pregnant women’s attitudes regarding acceptance of influenza vaccination have improved in the intervening years, and some evidence that safety concerns have actually increased.43 It may be that the important increases in uptake of influenza vaccination among pregnant women can primarily be attributed to improved vaccine delivery by obstetricians.
Barriers to stocking and administering vaccines to pregnant women by obstetricians continue to be primarily financial, particularly for inadequate reimbursement for vaccine purchase and vaccine administration. Although the questions were asked differently, these findings are similar to the findings of Power et al from 2007, where roughly half of physicians endorsed statements regarding financial barriers. These findings are discouraging given the extensive efforts to increase access to adult vaccination among vaccine advocates and the stipulations of the Affordable Care Act that all Advisory Committee on Immunization Practices (ACIP) recommended vaccines be covered by non-grandfathered insurance companies with no copay (first dollar coverage). In contrast to the Powers study, though, attitudinal barriers among obstetricians themselves have essentially disappeared. The most direct comparison between the two studies is regarding safety: in 2007, 32% of respondents agreed that “we still don’t know enough about the effects of vaccines on the fetus to administer them safely in pregnancy.” In this study, obstetricians’ concern about the safety of vaccines in pregnancy was reported as ‘not a barrier’ by 88% of respondents. On the other hand, patient attitudinal concerns regarding vaccination in pregnancy was a significant perceived barrier for many ob-gyns, suggesting the need for ongoing efforts to improve pregnant women’s acceptance of vaccination.
The findings of the multivariable analysis offer some insight into reasons some physicians are not following the recommendation to administer influenza and Tdap vaccines to pregnant women, and may provide a better understanding of some of the barriers. The finding that more women than men administer these vaccines is consistent with prior literature showing that female physicians are more likely to adhere to clinical guidelines44–46 and provide preventive care more often.47–54 The finding that those administering both vaccines perceive more patient barriers may be related to the fact that because they are administering both vaccines, they are experiencing more patient resistance than those physicians who do not. Physicians who are in private practice were also less likely to administer these vaccines. This may be because hospitals and larger systems are better equipped to overcome the financial and logistical barriers to vaccine delivery. Such organizations likely already stock these vaccines for use in other settings, and they also often have the infrastructure necessary to implement system-level changes that strongly promote guideline adherence. One explanation for why physicians who do not stock both vaccines report greater financial barriers is because these may be perceived barriers more than they are true barriers. Ob-gyns who stock and administer these vaccines report fewer financial barriers; this fact should offer some encouragement to those currently not stocking these vaccines. Providers may also benefit from increased use of available resources. For example, more than half of ob-gyns rarely or never use the ACOG immunization toolkits. These toolkits have extensive information on vaccine financing and coding that could address some of the perceived financial barriers.55
Ob-gyns reported infrequent use of several evidence-based strategies for increasing vaccination uptake. While a sizable proportion reported use of standing orders, more for influenza vaccine than for Tdap, few reported using reminder/recall or an IIS. An IIS is a confidential, population-based, computerized database that records all immunization doses administered by participating providers to persons residing within a given geopolitical area.56,57 Infrequent use of IISs by ob-gyns is not surprising given the infrequent use by other specialties delivering primary care primarily to adult patients, such as internists,58 and the lack of infrastructure for supporting adult vaccination recording in IISs in many states.57 More frequent use of IIS by ob-gyns is a worthy goal, but there are many systemic barriers that will need to be resolved prior to widespread adoption. It may also be that for the routine vaccines of pregnancy, influenza and Tdap, ob-gyns do not perceive a need to use an IIS. Similarly, reminder/recall for these routine vaccines may be perceived as unnecessary since patients are seen so frequently during a pregnancy. However, for other vaccines indicated for some pregnant women, systematic reminder/recall may be a well-suited strategy as it could take the need for determining eligibility for infrequently delivered vaccines out of the busy provider’s hands. Because there does not appear to be a common systematic way for determining eligibility for these other vaccines, though, frequent use of reminder/recall by ob-gyns – similar to adoption of IIS – is unlikely to happen anytime soon.
This study has several strengths and limitations. It was from a nationally representative sample of ob-gyns, and there was a high response rate. However, respondents’ attitudes and practices may have differed from non-respondents, and network physicians may differ from physicians overall, although prior work suggests not.34 Also, this survey assessed practices of only ob-gyns, so other prenatal care providers such as family physicians and nurse midwives are not represented. Future work should examine maternal vaccination practices among these providers, as their professional organizations strongly endorse vaccination of pregnant women, yet there have been no recent national surveys assessing their practices and attitudes. Finally, this study assessed reported practices; actual practices were not observed.
Ob-gyns have made great strides in the delivery of influenza and Tdap vaccines in pregnancy in recent years. However, significant gaps remain: A quarter of ob-gyns are still not stocking Tdap vaccine leaving many infants vulnerable to pertussis, and for vaccines other than influenza and Tdap that may be indicated in pregnancy, most ob-gyns are not assessing eligibility and even fewer are stocking these vaccines. To address these gaps, novel approaches may be needed, such as a program for pregnant women similar to the Vaccines for Children (VFC) program, which has been instrumental in increasing uptake of childhood vaccines. Future work should address the feasibility of more complete vaccine delivery in ob-gyn settings, exploring and addressing potential barriers, and offering sustainable solutions.
Acknowledgments
The authors would like to thank Erin McBurney for her assistance in preparing the manuscript. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. This investigation was funded by the Centers for Disease Control and Prevention and administered through the Rocky Mountain Prevention Research Center, University of Colorado Anschutz Medical Campus (Grant #1U01IP000849). Dr. O’Leary conceptualized and designed the study, contributed to the data collection instrument design, drafted the initial and final manuscript; Drs. Kempe, Riley, Allison, and Hurley and Ms. Albert, Fisher, Jiles, and Lindley assisted in study design and creation of the data collection instrument and reviewed and revised the manuscript; Dr. Crane conceptualized and designed the study, designed the data collection instrument, and reviewed and revised the manuscript; Ms Beaty contributed to the study design, carried out the initial and further analyses, and reviewed and revised the manuscript; Dr. Brtnikova and Ms. Collins contributed to the study design and data collection instrument design, coordinated and supervised all data collection, and reviewed and revised the manuscript. All authors approved the final manuscript as submitted. Portions of this work were presented at the National Immunization Conference, Atlanta, Georgia, September 2016 and IDWeek, New Orleans, Louisiana, October 2016. No financial disclosures were reported by the authors of this paper.
Footnotes
CONFLICT OF INTEREST:
No authors have conflicts of interest to disclose.
FINANCIAL DISCLOSURE:
No authors have financial relationships relevant to this article to disclose.
References
- 1.Stupiansky NW, Zimet GD, Cummings T, Fortenberry JD, Shew M. Accuracy of self-reported human papillomavirus vaccine receipt among adolescent girls and their mothers. J Adolesc Health. 2012;50(1):103–105. doi: 10.1016/j.jadohealth.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Creanga AA, Johnson TF, Graitcer SB, et al. Severity of 2009 pandemic influenza A (H1N1) virus infection in pregnant women. Obstet Gynecol. 2010;115(4):717–726. doi: 10.1097/AOG.0b013e3181d57947. [DOI] [PubMed] [Google Scholar]
- 3.Hartert TV, Neuzil KM, Shintani AK, et al. Maternal morbidity and perinatal outcomes among pregnant women with respiratory hospitalizations during influenza season. Am J Obstet Gynecol. 2003;189(6):1705–1712. doi: 10.1016/s0002-9378(03)00857-3. [DOI] [PubMed] [Google Scholar]
- 4.Louie JK, Acosta M, Jamieson DJ, Honein MA, California Pandemic Working G. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med. 2010;362(1):27–35. doi: 10.1056/NEJMoa0910444. [DOI] [PubMed] [Google Scholar]
- 5.Neuzil KM, Reed GW, Mitchel EF, Simonsen L, Griffin MR. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol. 1998;148(11):1094–1102. doi: 10.1093/oxfordjournals.aje.a009587. [DOI] [PubMed] [Google Scholar]
- 6.Ampofo K, Gesteland PH, Bender J, et al. Epidemiology, complications, and cost of hospitalization in children with laboratory-confirmed influenza infection. Pediatrics. 2006;118(6):2409–2417. doi: 10.1542/peds.2006-1475. [DOI] [PubMed] [Google Scholar]
- 7.Izurieta HS, Thompson WW, Kramarz P, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med. 2000;342(4):232–239. doi: 10.1056/NEJM200001273420402. [DOI] [PubMed] [Google Scholar]
- 8.Mullooly JP, Barker WH. Impact of type A influenza on children: a retrospective study. Am J Public Health. 1982;72(9):1008–1016. doi: 10.2105/ajph.72.9.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortese MM, Baughman AL, Zhang R, Srivastava PU, Wallace GS. Pertussis hospitalizations among infants in the United States, 1993 to 2004. Pediatrics. 2008;121(3):484–492. doi: 10.1542/peds.2007-1393. [DOI] [PubMed] [Google Scholar]
- 10.Vitek CR, Pascual FB, Baughman AL, Murphy TV. Increase in deaths from pertussis among young infants in the United States in the 1990s. Pediatr Infect Dis J. 2003;22(7):628–634. doi: 10.1097/01.inf.0000073266.30728.0e. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women–Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep. 2013;62(7):131–135. [PMC free article] [PubMed] [Google Scholar]
- 12.Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR RecommRep. 2004;53(RR-6):1–40. [PubMed] [Google Scholar]
- 13.Dabrera G, Zhao H, Andrews N, et al. Effectiveness of seasonal influenza vaccination during pregnancy in preventing influenza infection in infants, England, 2013/14. Euro Surveill. 2014;19(45):20959. doi: 10.2807/1560-7917.es2014.19.45.20959. [DOI] [PubMed] [Google Scholar]
- 14.Eick AA, Uyeki TM, Klimov A, et al. Maternal influenza vaccination and effect on influenza virus infection in young infants. Arch Pediatr Adolesc Med. 2011;165(2):104–111. doi: 10.1001/archpediatrics.2010.192. [DOI] [PubMed] [Google Scholar]
- 15.Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359(15):1555–1564. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- 16.Poehling KA, Szilagyi PG, Staat MA, et al. Impact of maternal immunization on influenza hospitalizations in infants. Am J Obstet Gynecol. 2011;204(6 Suppl 1):S141–148. doi: 10.1016/j.ajog.2011.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benowitz I, Esposito DB, Gracey KD, Shapiro ED, Vazquez M. Influenza vaccine given to pregnant women reduces hospitalization due to influenza in their infants. Clin Infect Dis. 2010;51(12):1355–1361. doi: 10.1086/657309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amirthalingam G, Andrews N, Campbell H, et al. Effectiveness of maternal pertussis vaccination in England: an observational study. Lancet. 2014;384(9953):1521–1528. doi: 10.1016/S0140-6736(14)60686-3. [DOI] [PubMed] [Google Scholar]
- 19.Winter K, Cherry JD, Harriman K. Effectiveness of prenatal Tdap vaccination on pertussis severity in infants. Clin Infect Dis. 2016 doi: 10.1093/cid/ciw633. [DOI] [PubMed] [Google Scholar]
- 20.Winter K, Nickell S, Powell M, Harriman K. Effectiveness of prenatal versus postpartum Tdap vaccination in preventing infant pertussis. Clin Infect Dis. 2016 doi: 10.1093/cid/ciw634. [DOI] [PubMed] [Google Scholar]
- 21.Munoz FM, Greisinger AJ, Wehmanen OA, et al. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol. 2005;192(4):1098–1106. doi: 10.1016/j.ajog.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 22.Nordin JD, Kharbanda EO, Benitez GV, et al. Maternal safety of trivalent inactivated influenza vaccine in pregnant women. Obstet Gynecol. 2013;121(3):519–525. doi: 10.1097/AOG.0b013e3182831b83. [DOI] [PubMed] [Google Scholar]
- 23.Kharbanda EO, Vazquez-Benitez G, Lipkind HS, et al. Evaluation of the association of maternal pertussis vaccination with obstetric events and birth outcomes. JAMA. 2014;312(18):1897–1904. doi: 10.1001/jama.2014.14825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berenson AB, Hirth JM, Rahman M, Laz TH, Rupp RE, Sarpong KO. Maternal and infant outcomes among women vaccinated against pertussis during pregnancy. Hum Vaccin Immunother. 2016;12(8):1965–1971. doi: 10.1080/21645515.2016.1157241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munoz FM, Bond NH, Maccato M, et al. Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants: a randomized clinical trial. JAMA. 2014;311(17):1760–1769. doi: 10.1001/jama.2014.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shakib JH, Korgenski K, Sheng X, Varner MW, Pavia AT, Byington CL. Tetanus, diphtheria, acellular pertussis vaccine during pregnancy: pregnancy and infant health outcomes. J Pediatr. 2013;163(5):1422–1426. e1421–1424. doi: 10.1016/j.jpeds.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sukumaran L, McCarthy NL, Kharbanda EO, et al. Association of Tdap Vaccination With Acute Events and Adverse Birth Outcomes Among Pregnant Women With Prior Tetanus-Containing Immunizations. JAMA. 2015;314(15):1581–1587. doi: 10.1001/jama.2015.12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding H, Black CL, Ball S, et al. Influenza vaccination coverage among pregnant women–United States, 2014–15 influenza season. MMWR Morb Mortal Wkly Rep. 2015;64(36):1000–1005. doi: 10.15585/mmwr.mm6436a2. [DOI] [PubMed] [Google Scholar]
- 29.Ding H, Black CL, Ball S, et al. Influenza vaccination coverage among pregnant women–United States, 2013–14 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63(37):816–821. [PMC free article] [PubMed] [Google Scholar]
- 30.Kharbanda EO, Vazquez-Benitez G, Lipkind HS, et al. Maternal Tdap vaccination: Coverage and acute safety outcomes in the vaccine safety datalink, 2007–2013. Vaccine. 2016;34(7):968–973. doi: 10.1016/j.vaccine.2015.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koepke R, Kahn D, Petit AB, et al. Pertussis and Influenza Vaccination Among Insured Pregnant Women - Wisconsin, 2013–2014. MMWR Morb Mortal Wkly Rep. 2015;64(27):746–750. [PMC free article] [PubMed] [Google Scholar]
- 32.O’Leary ST, Pyrzanowski J, Brewer SE, et al. Influenza and Pertussis Vaccination Among Pregnant Women and Their Infants’ Close Contacts: Reported Practices and Attitudes. Pediatr Infect Dis J. 2015;34(11):1244–1249. doi: 10.1097/INF.0000000000000873. [DOI] [PubMed] [Google Scholar]
- 33.Swamy GK, Heine RP. Vaccinations for pregnant women. Obstet Gynecol. 2015;125(1):212–226. doi: 10.1097/AOG.0000000000000581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crane LA, Daley MF, Barrow J, et al. Sentinel physician networks as a technique for rapid immunization policy surveys. Eval Health Prof. 2008;31(1):43–64. doi: 10.1177/0163278707311872. [DOI] [PubMed] [Google Scholar]
- 35.Hurley LP, Bridges CB, Harpaz R, et al. U.S. physicians’ perspective of adult vaccine delivery. Ann Intern Med. 2014;160(3):161. doi: 10.7326/M13-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Leary ST, Crane LA, Wortley P, et al. Adherence to expanded influenza immunization recommendations among primary care providers. The Journal of pediatrics. 2012;160(3):480–486. doi: 10.1016/j.jpeds.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 37.O’Leary ST, Stokley S, Crane LA, et al. Influenza vaccination in the 2009–2010 pandemic season: the experience of primary care physicians. Prev Med. 2012;55(1):68–71. doi: 10.1016/j.ypmed.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 38.Dillman DA, Smyth JD, Chistian LM. Internet, Phone, Mail, and Mixed-Mode Surveys: The Tailored Design Method. 4th. New York, NY: John Wiley & Sons, Inc; 2014. [Google Scholar]
- 39.McMahon SR, Iwamoto M, Massoudi MS, et al. Comparison of e-mail, fax, and postal surveys of pediatricians. Pediatrics. 2003;111(4 Pt 1):e299–303. doi: 10.1542/peds.111.4.e299. [DOI] [PubMed] [Google Scholar]
- 40.Power ML, Leddy MA, Anderson BL, Gall SA, Gonik B, Schulkin J. Obstetrician-gynecologists’ practices and perceived knowledge regarding immunization. Am J Prev Med. 2009;37(3):231–234. doi: 10.1016/j.amepre.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 41.Link-Gelles R, Chamberlain AT, Schulkin J, et al. Missed opportunities: a national survey of obstetricians about attitudes on maternal and infant immunization. Matern Child Health J. 2012;16(9):1743–1747. doi: 10.1007/s10995-011-0936-0. [DOI] [PubMed] [Google Scholar]
- 42.Lu PJ, Santibanez TA, Williams WW, et al. Surveillance of influenza vaccination coverage–United States, 2007–08 through 2011–12 influenza seasons. MMWR Surveill Summ. 2013;62(4):1–28. [PubMed] [Google Scholar]
- 43.Chamberlain AT, Berkelman RL, Ault KA, Rosenberg ES, Orenstein WA, Omer SB. Trends in reasons for non-receipt of influenza vaccination during pregnancy in Georgia, 2004–2011. Vaccine. 2016;34(13):1597–1603. doi: 10.1016/j.vaccine.2016.01.058. [DOI] [PubMed] [Google Scholar]
- 44.Kim C, McEwen LN, Gerzoff RB, et al. Is physician gender associated with the quality of diabetes care? Diabetes Care. 2005;28(7):1594–1598. doi: 10.2337/diacare.28.7.1594. [DOI] [PubMed] [Google Scholar]
- 45.Berthold HK, Gouni-Berthold I, Bestehorn KP, Bohm M, Krone W. Physician gender is associated with the quality of type 2 diabetes care. J Intern Med. 2008;264(4):340–350. doi: 10.1111/j.1365-2796.2008.01967.x. [DOI] [PubMed] [Google Scholar]
- 46.Baumhakel M, Muller U, Bohm M. Influence of gender of physicians and patients on guideline-recommended treatment of chronic heart failure in a cross-sectional study. Eur J Heart Fail. 2009;11(3):299–303. doi: 10.1093/eurjhf/hfn041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andersen MR, Urban N. Physician gender and screening: do patient differences account for differences in mammography use? Women Health. 1997;26(1):29–39. doi: 10.1300/J013v26n01_03. [DOI] [PubMed] [Google Scholar]
- 48.Frank E, Dresner Y, Shani M, Vinker S. The association between physicians’ and patients’ preventive health practices. CMAJ. 2013;185(8):649–653. doi: 10.1503/cmaj.121028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frank E, Harvey LK. Prevention advice rates of women and men physicians. Arch Fam Med. 1996;5(4):215–219. doi: 10.1001/archfami.5.4.215. [DOI] [PubMed] [Google Scholar]
- 50.Franks P, Bertakis KD. Physician gender, patient gender, and primary care. J Womens Health (Larchmt) 2003;12(1):73–80. doi: 10.1089/154099903321154167. [DOI] [PubMed] [Google Scholar]
- 51.Franks P, Clancy CM. Physician gender bias in clinical decisionmaking: screening for cancer in primary care. Med Care. 1993;31(3):213–218. doi: 10.1097/00005650-199303000-00003. [DOI] [PubMed] [Google Scholar]
- 52.Kruger J, Shaw L, Kahende J, Frank E. Health care providers’ advice to quit smoking, National Health Interview Survey, 2000, 2005, and 2010. Prev Chronic Dis. 2012;9:E130. doi: 10.5888/pcd9.110340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lurie N, Slater J, McGovern P, Ekstrum J, Quam L, Margolis K. Preventive care for women. Does the sex of the physician matter? N Engl J Med. 1993;329(7):478–482. doi: 10.1056/NEJM199308123290707. [DOI] [PubMed] [Google Scholar]
- 54.Smith AW, Borowski LA, Liu B, et al. U.S. primary care physicians’ diet-, physical activity-, and weight-related care of adult patients. Am J Prev Med. 2011;41(1):33–42. doi: 10.1016/j.amepre.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Immunization Resources for Obstetrician-Gynecologists: A Comprehensive Toolkit. Online Reference. 2013 Available at: https://www.acog.org/-/media/Departments/Immunization/ImmunizationToolkit.pdf. Accessed February 20, 2017.
- 56.Centers for Disease Control and Prevention. About Immunization Information Systems, What is IIS? 2016 https://www.cdc.gov/vaccines/programs/iis/about.html. Accessed August 21, 2017.
- 57.Centers for Disease Control and Prevention. Progress in Immunization Information Systems - United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62(49):1005–1008. [PMC free article] [PubMed] [Google Scholar]
- 58.Kempe A, Hurley LP, Cardemil CV, et al. Use of Immunization Information Systems in Primary Care. Am J Prev Med. 2017;52(2):173–182. doi: 10.1016/j.amepre.2016.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]


