Abstract
Background
Although conventional wisdom suggests that differences in patient risk profiles drive variability in postoperative pneumonia rates after coronary artery bypass grafting (CABG), this teaching has yet to be empirically tested. We determined to what extent patient risk factors account for hospital variation in pneumonia rates.
Methods
We studied 324,085 patients undergoing CABG between July 1, 2011, and December 31, 2013, across 998 hospitals using The Society of Thoracic Surgeons Adult Cardiac Database. We developed 5 models to estimate our incremental ability to explain hospital variation in pneumonia rates. Model 1 contained patient demographic characteristics and admission status, while Model 2 added patient risk factors. Model 3 added measures of pulmonary function, Model 4 added measures of cardiac anatomy and function and medications, and Model 5 further added measures of intraoperative and postoperative care.
Results
Although 9,175 patients (2.83%) experienced pneumonia, the median estimated distribution of pneumonia rates across hospitals was 2.5% (25th to 75th percentile: 1.5% to 4.0%). Wide variability in pneumonia rates was evident, with some hospitals having rates more than 6 times higher than others (10th to 90th percentile: 1.0% to 6.1%). Among all five models, Model 2 accounted for the most variability at 4.24%. In total, 2.05% of hospital variation in pneumonia rates was explained collectively by traditional patient factors, leaving 97.95% of variation unexplained.
Conclusions
Our findings suggest that patient risk profiles only account for a fraction of hospital variation in pneumonia rates; enhanced understanding of other contributory factors (eg, processes of care) is required to lessen the likelihood of such nosocomial infections.
Hospital-acquired infections are among the most common type of complications after coronary artery bypass grafting (CABG) operation [1]. Patients presenting postoperatively with such infections are more likely to experience major morbidity and death [2-4]. Although patients may be exposed to many infections in this setting, pneumonia is the most prevalent, affecting 3% of patients [4].
Prior work has documented center-level variability in rates of postoperative pneumonia [4, 5]. Conventional wisdom suggests that differences in patient risk profiles are the predominant driver of variability in postoperative pneumonia after CABG operation. Nonetheless, center-level variation in pneumonia rates persists even after adjusting for patient risk [5]. Variation in pneumonia rates may be attributable in part to discrete and to potentially modifiable practices (eg, red blood cell transfusion rates, duration of intubation, antibiotic management). Although many risk factors for developing postoperative pneumonia have been identified, factors helping explain center-level differences in postoperative pneumonia rates have not been well elucidated.
We determined the contribution of patient and modifiable factors before, during, and after operations in explaining hospital variation in pneumonia rates. We hypothesized that variation in center-level pneumonia rates may be explained by preoperative presentation, as well as intraoperative and postoperative care.
Patients and Methods
The Society of Thoracic Surgeons Adult Cardiac Surgery Database
We included 365,686 patients undergoing isolated CABG operation between July 1, 2011, and December 31, 2013, at 1,084 hospitals participating in The Society of Thoracic Surgeons Adult Cardiac Surgery Database (STS-ACSD; version 2.73). We excluded patients undergoing cardiac operation for infectious endocarditis, heart transplantation, or ventricular assist device insertion or removal, and patients missing any of the following data fields: (1) pneumonia, (2) sepsis, (3) deep sternal wound infection, (4) mediastinitis, (5) harvest or cannulation site infection, and (6) thoracotomy. After exclusions, 324,085 patients, representing 998 hospitals, were available for subsequent analysis (Fig 1).
Fig 1.
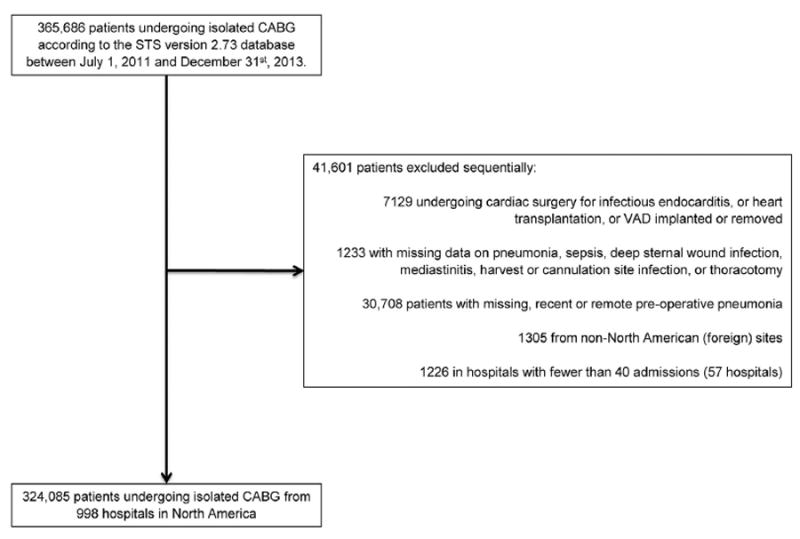
Selection criteria schema for coronary artery bypass grafting (CABG) patients from The Society of Thoracic Surgeons Adult Cardiac Surgery Database (STS-ACSD). (VAD = ventricular assist device.)
Study Variables
The primary outcome for this analysis was the postoperative development of pneumonia. The STS-ACSD bases pneumonia diagnosis on laboratory findings (eg, positive sputum culture results from transtracheal fluid, bronchial washings, or both), radiologic evidence (eg, chest roentgenogram diagnostic of pulmonary infiltrates), or both.
Rates of pneumonia were compared across hospitals based on adjusted postoperative pneumonia rates (see model 1 below).
Statistical Analysis
Continuous variables are presented as means, medians (25th, 75th percentile), and differences compared using the Wilcoxon rank-sum test. Categorical variables are presented as counts and percentages with differences being compared using the Pearson χ2 test. To ensure that hospitals did not drop out of the analysis during the sequential modeling process, we performed median and mode imputation for continuous and categorical covariates, respectively.
We followed the approach of Xian and colleagues [6] to quantify the degree to which demographic characteristics, risk factors, pulmonary function, cardiac anatomy and function, medications and laboratory findings, and intraoperative and postoperative care influence hospital-level postoperative pneumonia variability. Therefore, a series of multivariable hierarchical logistic regression models, with a random intercept for hospital, were built. The first model we considered was the unadjusted (or empty) model (Model 0) that contained only hospital-specific random intercepts and no covariates. Then, we fit subsequent models by incrementally adjusting for demographic characteristics, body mass index, and some patients’ characteristics at the time of presentation (Model 1); additional risk factors (Model 2); pulmonary function (Model 3); cardiac anatomy and function and medications and laboratory findings (Model 4); and intraoperative and postoperative care (Model 5) (Supplemental Table 1). The list of variables explored but not incorporated into our models are included in Supplemental Table 2.
The empty Model 0 assumes that the rate of pneumonia is similar for every patient and estimates the hospital- and patient-level variations of pneumonia. Model 1 consisted of patient covariates, including admission source and status, preoperative length of stay, age, race, sex, and body mass index. We calculated risk-adjusted hospital postoperative pneumonia rates for each hospital using estimated hospital-specific variables from the respective model (Model 1). These rates were defined as the ratio of predicted-to-expected postoperative pneumonia, multiplied by the overall unadjusted postoperative pneumonia rate [7]. For descriptive purposes, we further divided the hospitals into quartiles according to adjusted postoperative pneumonia rates.
Model 2 consisted of covariates in Model 1 plus risk factors. Model 3 consisted of covariates in Model 2 plus measures of pulmonary function. Model 4 consisted of covariates in Model 3 plus measures of cardiac anatomy and function, and medications and laboratory findings. Model 5 consisted of Model 4 covariates plus intraoperative and postoperative care. Adding intraoperative and postoperative care in Model 5 allowed us to capture any variations that may be explained by these additional covariates beyond and above those explain by the covariates in Model 4.
For two sequential consecutive models A and B, we estimated the proportional change in variance (PCV) as the ratio
where estimates of the variances VA and VB, respectively, for the initial model and the model with additional covariates, are calculated using estimates of random effect variances of their respective sequential models (ie, variation in log-odds attributable to between-hospital differences) as indicated by Merlo and colleagues [8, 9]. To limit sampling variation due to hospitals with a small number of patients, we reported estimated distributions rather than observed distributions of postoperative pneumonia rates. Based on the modus operandi of Xian and colleagues [6], for each set of adjustment covariates we used a hierarchical model to estimate the distribution of hospital rates of postoperative pneumonia after subtracting out the effect of random sampling variation. In that regard, we typically assumed that the log-odds for random hospital were normally distributed with mean equal to the intercept and variance equal to the random effect variance. We first estimated the mean and the variance of the distribution of postoperative pneumonia rates for the unadjusted model using the corresponding variable estimates and transformed from the log-odds scale to the probability scale. Then, for the subsequent models M1 to M5, we replaced the random effect variance along with new estimates based on the adjusted models to derive their corresponding mean and variance. Finally, we overlaid on a single plot the estimated distribution of hospital-specific postoperative pneumonia rates for each hospital to represent the relative reduction in variation (Fig 2), using previously published methods [6].
Fig 2.
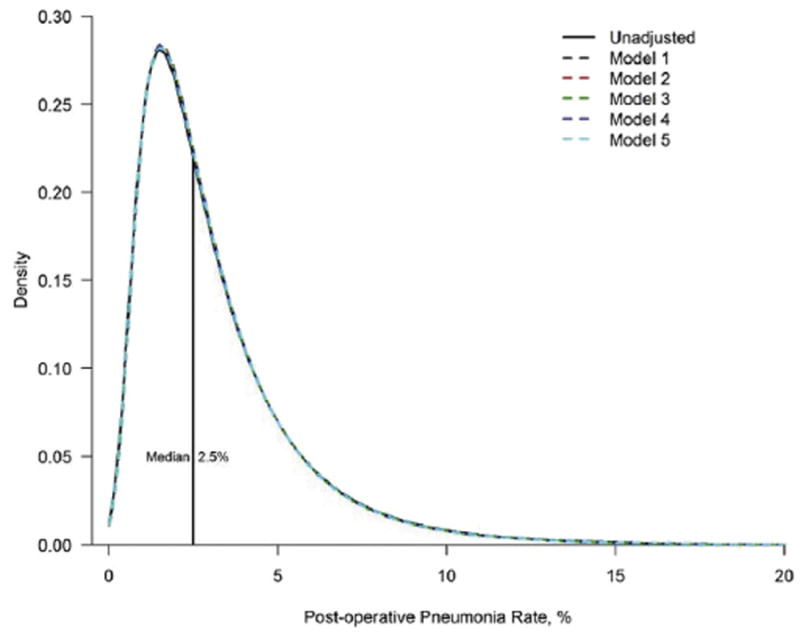
Estimated distribution of hospital-specific postoperative pneumonia rates. The area between any two points on the x-axis represents the proportion of hospitals within that range.
Statistical analyses were performed using SAS version 9.3 (SAS Institute, Inc, Cary, NC) and R version 2.15.2 (R Foundation for Statistical Computing, Vienna, Austria). The two-tailed tests were considered significant at p less than 0.05. This study was approved by the Duke University Health System Institutional Review Board.
Results
Among 324,085 isolated CABG patients across 998 hospitals (median patients per hospital 255, interquartile range [IQR]: 145 to 419), 9,175 patients (2.83%) experienced pneumonia (Table 1). The median estimated distribution of pneumonia rates across hospitals was 2.5% (IQR: 1.5% to 4.0%), with some hospitals having rates more than six times higher than others (10th to 90th percentile: 1.0% to 6.1%).
Table 1.
Distribution of Postoperative Pneumonia Rates According to Quartiles of Hospital Risk-Adjusted Postoperative Pneumonia Rates
| Overall | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
|---|---|---|---|---|---|
| Median rate, %a | 1.40 | 2.36 | 3.55 | 6.05 | |
| Patients, n | 324,085 | 87,243 | 80,078 | 79,917 | 76,847 |
| Hospitals, n | 998 | 249 | 250 | 250 | 249 |
| Observed rate, % | 2.83 | 0.55 | 1.75 | 3.12 | 6.63 |
Adjusted for patient-level characteristics, including admission source, admission status, demographic characteristics (age, race, and sex), body mass index, and preoperative length of stay.
Differences in case mix and processes of care across increasing quartiles of center-based–adjusted pneumonia rates are displayed in Supplemental Table 1. More than 50% of patients were urgent, median age was 65 years, 74.36% were men, median white blood cell count before operation was 7.60×109/L, and 2.31% were receiving immunosuppressive treatment. For risk factors, 1.47% were on home oxygen, 9.13% were using oral bronchodilator therapy, 32.73% were cigarette smokers, 9.80% had moderate-to-severe chronic lung disease, 2.93% underwent previous CABG, 32.75% had left main disease 50% or greater, 75.40% had three-vessel disease, and 15.96% had congestive heart failure. Intraoperative and postoperative characteristics, also across increasing quartiles of adjusted pneumonia rates, are also shown in Supplemental Table 1. The median bypass and crossclamp times were 90 and 64 minutes, respectively. Almost one-half (49.88%) of patients received transfusions; 9.12% had prolonged ventilation. Most patients had the appropriate antibiotic selection (97.93%), timing (98.96%), and discontinuation (97.70%) of antibiotics.
Small differences in patient demographic, preoperative, intraoperative, and postoperative characteristics were noted across hospital quartiles. (Supplemental Table 1). Measures of pulmonary function were qualitatively similar across hospital quartiles. Mitral valve disease was qualitatively similar in the top three quartiles but lowest in the fourth quartile (35.72%, 38.57%, 36.50%, 29.15% for quartile 4 to quartile 1, respectively; p < 0.0001). Patients in higher quartiles had longer median operating room duration (308, 303, 295, 289 minutes for quartile 4 to quartile 1, respectively; p < 0.0001) and increased rate of intraoperative or postoperative transfusions (55.48%, 49.11%, 47.74%, 47.62% for quartile 4 to quartile 1, respectively; p < 0.0001), although similar median lowest intraoperative hematocrit. Bypass and cross-clamp durations were similar across quartiles. Patients in the highest quartile were more likely to experience prolonged ventilation (11.59%, 9.73%, 8.38%, 7.09% for quartile 4 to quartile 1, respectively; p < 0.0001). Similar antibiotic selection, timing, and discontinuation were observed.
The estimated median odds ratios (MORs) remained almost constant and substantially high from Model 0 to Model 5, oscillating between 2.00 and 2.03. It was equal to 2.03 in Model 0, 2.02 in Model 1, 2.00 in Model 2, 2.01 in models 3 and 4, and equal to 2.02 in Model 5. The MOR defined the median value of the distribution of odds ratios that would be obtained if we randomly picked two patients with identical covariates but treated in two different hospitals and comparing their propensity to acquire pneumonia from the higher risk hospital with the one from a lower risk hospital [10]. Therefore, an MOR of 2.00 indicates substantial heterogeneity between hospitals in rates of pneumonia. The propensity by a patient to acquire pneumonia varies enormously from one hospital to another, above, and beyond that of patient demographic characteristics and risk factors.
Estimated distribution of pneumonia rates corresponding to each sequential model is displayed in Figure 3. A 1.95% reduction in PCV(M0, M1) was observed when comparing model 1 adjusted for demographic characteristics with the unadjusted model (ie, model 0). Compared with the unadjusted model, incorporation of patient risk factors was associated with a 4.24% reduction in PCV. When all models are considered collectively, 2.05% of hospital variation in pneumonia rates was attributable to demographic characteristics, risk factors, pulmonary function, cardiac anatomy and function, medications and laboratory findings, and intraoperative and postoperative care.
Fig 3.
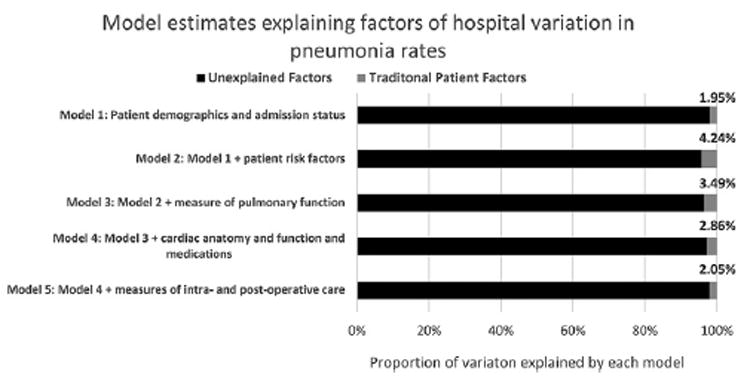
Model estimates explaining factors of hospital variation in pneumonia rates.
An incremental reduction of 2.34% in pneumonia variation was observed after adjusting for risk factors. Further incorporation of pulmonary function was associated with a 0.79% increase in variation. Similar findings were observed when incorporating cardiac anatomy and function, medications and laboratory findings, and intraoperative and postoperative care into the model.
Comment
We determined the contribution of preoperative, intraoperative, and postoperative patient and modifiable factors in explaining hospital variation in pneumonia rates. Our results contradict conventional thinking, namely that variation in pneumonia rates across hospitals is driven by differences in baseline risk factors. We found that only 2.05% of hospital variation is explained when accounting for traditional measures of patient risk and quality. Our findings also suggest that traditional measures do not incrementally explain variation in hospital pneumonia rates. Even after accounting for these known factors, only 2.05% of center-level variability in postoperative pneumonia rates could be explained. Future areas of investigation should focus on enhancing our understanding of other factors, including processes of care that may lessen the likelihood of such nosocomial infections.
We cannot rule out systematic bias in reporting pneumonia rates. Nonetheless, STS-ACSD participants are subject to random external audits. Furthermore, our reported rate of pneumonia is similar to other series [4]. We do not report the ascertainment of all pneumonia, namely those identified after discharge. Any underestimation in the true rate will not inherently bias our findings, given that STS-ACSD participants uniformly adhere to the same data elements. Our study was observational; thus, it is subject to unmeasured confounding (eg, socioeconomic status).
Recent attention has focused on health care–associated infections, given their relationship with patient safety and expenditures. The Centers for Medicare and Medicaid Services implemented a policy in 2008 to withhold payment for preventable conditions [11]. The Agency for Healthcare Research and Quality estimates that a 17% reduction in infections occurred between 2010 and 2013, including 50,374 averted deaths and $11.98 billion in savings [12]. Although the focus of this effort was broader than cardiac operations, identifying modifiable practices associated with preventable infections was a likely contributor [13].
Prior reports have contributed to our understanding about the epidemiologic factors underlying infections after CABG. Although not including pneumonia, Fowler and colleagues [3] reported a 3.51% rate of major infections among 331,429 patients undergoing any CABG between 2002 and 2003. Shih and colleagues [4] investigated hospital-level variability in infection rates among 20,896 isolated CABG patients between 2009 and 2012 at Michigan hospitals. Pneumonia accounted for most infections (overall rate 5.1%) across low-, medium-, and high-rate hospitals. Similar findings were noted using STS-ACSD data [5].
Investigators have developed prediction models using a variety of cardiac surgical populations and methodologic approaches [14-16]. These models often include patient-level characteristics (eg, age, body mass index, smoking history, white blood cell count, acuity, preoperative length of stay, home oxygen use, history of pneumonia, moderate or severe chronic lung disease), although potentially modifiable intraoperative (eg, blood transfusions) and postoperative (eg, mechanical ventilation longer than 1 day) factors as well [14]. Even after accounting for these patient-level factors, nearly 96% of inter-hospital variation in pneumonia rates remains unexplained. Additional measures of cardiac anatomy, function, medications, infection control, and intraoperative and postoperative care further decrease our ability to account for hospital variation. These findings, although perhaps surprising, suggest that other explanatory factors exist (ie, unmeasured confounding).
Donabedian [17] defined a hospital’s performance as the by-product of processes of care and a hospital’s structure. Our findings validate this framework, given that only 2% of hospital-level variation in pneumonia rates are explained by differences in patient factors. Although counter-intuitive for some, opportunities may exist to diminish unwarranted variation in pneumonia rates by identifying processes and systems of care that increase a patient’s risk of this adverse sequelae.
These processes and systems begin with preoperative optimization, or prehabilitation. Preoperative decolonization therapy using chlorhexidine body washes, intranasal mupirocin, or both, reduces surgical site infections [18]. Preoperative optimization (eg, abstinence of cigarette smoking before the operation, use of an incentive spirometer, and walking 1 to 3 miles per day) has been well described in patients preparing for esophagectomy for reducing pneumonia [19]. Patients achieving more than 1 week of preoperative nonsmoking have 10-fold increased odds of continued nonsmoking 1 year after CABG [20] and a considerable reduced risk of mortality at 5 years [21]. The Michigan Surgical Home and Optimization Program was developed to provide a structured prehabilitation routine, including smoking cessation, with 79% of enrollees remaining smoke-free at first follow-up visit [22]. Structured smoking cessation programs may help reduce pneumonia rates for elective CABG patients.
Intraoperative strategies for decreasing pneumonia rates may include lung protective ventilation and the use of low tidal volumes and positive end-expiratory pressure. The increased importance of these practices has been identified for cardiac operations because of the relatively longer mechanical ventilation periods, frequent comorbidities, and proinflammatory cofactors (eg, cardiopulmonary bypass) [23]. Although intraoperative lung protective strategies benefit abdominal surgical patients, a standard lung protective ventilator strategy for cardiac operations has not yet been established [24]. A recent report showed that cardiac surgical patients receiving continuous aspiration of subglottic secretions (relative to a standard endotracheal tube) had lower 30-day mortality, median ventilation time, intensive care unit length of stay, and incidence of pneumonia [25]. A similar prospective analysis showed a lower incidence of ventilatorassociated pneumonia, cost of antibiotics, and days of ventilation [26].
In addition, implementation of fast-track extubation protocols with subsequent early extubation have been shown to reduce intensive care unit length of stay and prolonged ventilation. Prolonged ventilation occurred in 11.59% of patients in the highest pneumonia quartile in our analysis, compared with 7.09% in the lowest quartile. Reducing fluid retention with hemoconcentration and minimizing time on cardiopulmonary bypass may help achieve earlier extubation [27]. A recent Cochrane review suggested no evidence of benefit of incentive spirometry in reducing pulmonary complications; nonetheless, appropriately powered trials are warranted [28].
It is unclear what is driving differences in pneumonia rates across centers. Some have argued it is patient demographic characteristics (eg, older age), whereas others argue it is driven by risk factors, pulmonary function, or ischemic time. As such, we explored each of these using a statistical approach that has previously been used in the setting of acute myocardial infarction. This approach enables the investigators to quantify the unique contribution of each of these potential variables in explaining center-level differences in pneumonia rates.
A variety of more complex practices have been studied, and have identified that high-performing centers more often (1) have strong organizational leadership and (2) focus on adopting effective prevention activities [29, 30]. The use and incorporation of these practices, although not currently captured through clinical registries, may help to explain some of the observed differences in pneumonia rates.
A number of quality improvement collaboratives have emerged whose mission is to improve the quality and safety of cardiac operations. These collaboratives, which have had noteworthy successes [31, 32], often leverage benchmarking site visits to improve their understanding of processes of care that may help explain differences in performance. These efforts are well under way in Michigan through our statewide collaborative. We have embarked on site visits to help elucidate best practices that may be protective of postoperative pneumonia. A mixed methods approach, including site visiting, may help explain differences in pneumonia rates across hospitals. Early findings suggest that adoption of these practices may afford opportunities to reduce the rate of health care–associated infections.
In this large, nationally representative study, traditional measures of patient risk and quality explain only 2.05% of variation in pneumonia rates across hospitals. Our findings suggest the need to enhance our understanding of other factors that may lessen the likelihood of such nosocomial infections. These additional processes of care have been masked by our limited lens of analysis through national databases. Differences in outcomes and improvement in pneumonia rates would not be expected without changes in the way that we care for patients.
Supplementary Material
Acknowledgments
The authors wish to acknowledge the editorial review provided by Mark Cantrell. This project was supported by grant R01HS022535 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
Footnotes
Presented at the Sixty-third Annual Meeting of the Southern Thoracic Surgical Association, Naples, FL, Nov 9–12, 2016.
Dr Likosky discloses a financial relationship with the Agency for Healthcare Research & Quality.
The Supplemental Tables can be viewed in the online version of this article [http://dx.doi.org/10.1016/j.athoracsur.2017.08.012] on http://www.annalsthoracicsurgery.org.
References
- 1.Horvath KA, Acker MA, Chang H, et al. Blood transfusion and infection after cardiac surgery. Ann Thorac Surg. 2013;95:2194–201. doi: 10.1016/j.athoracsur.2012.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown PP, Kugelmass AD, Cohen DJ, et al. The frequency and cost of complications associated with coronary artery bypass grafting surgery: results from the United States Medicare program. Ann Thorac Surg. 2008;85:1980–6. doi: 10.1016/j.athoracsur.2008.01.053. [DOI] [PubMed] [Google Scholar]
- 3.Fowler VG, Jr, O’Brien SM, Muhlbaier LH, Corey GR, Ferguson TB, Peterson ED. Clinical predictors of major infections after cardiac surgery. Circulation. 2005;112:I358–65. doi: 10.1161/CIRCULATIONAHA.104.525790. [DOI] [PubMed] [Google Scholar]
- 4.Shih T, Zhang M, Kommareddi M, et al. Center-level variation in infection rates after coronary artery bypass grafting. Circ Cardiovasc Qual Outcomes. 2014;7:567–73. doi: 10.1161/CIRCOUTCOMES.113.000770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Likosky DS, Wallace AS, Prager RL, et al. Sources of variation in hospital-level infection rates after coronary artery bypass grafting: an analysis of The Society of Thoracic Surgeons Adult Heart Surgery Database. Ann Thorac Surg. 2015;100:1570–5. doi: 10.1016/j.athoracsur.2015.05.015. discussion 1575–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xian Y, Chen AY, Thomas L, et al. Sources of hospital-level variation in major bleeding among patients with non-stsegment elevation myocardial infarction: a report from the National Cardiovascular Data Registry (NCDR) Circ Cardiovasc Qual Outcomes. 2014;7:236–43. doi: 10.1161/CIRCOUTCOMES.113.000715. [DOI] [PubMed] [Google Scholar]
- 7.Shahian DM, Torchiana DF, Shemin RJ, Rawn JD, Normand SL. Massachusetts cardiac surgery report card: implications of statistical methodology. Ann Thorac Surg. 2005;80:2106–13. doi: 10.1016/j.athoracsur.2005.06.078. [DOI] [PubMed] [Google Scholar]
- 8.Merlo J, Chaix B, Ohlsson H, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health. 2006;60:290–7. doi: 10.1136/jech.2004.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merlo J, Yang M, Chaix B, Lynch J, Rastam L. A brief conceptual tutorial on multilevel analysis in social epidemiology: investigating contextual phenomena in different groups of people. J Epidemiol Community Health. 2005;59:729–36. doi: 10.1136/jech.2004.023929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsen K, Merlo J. Appropriate assessment of neighborhood effects on individual health: integrating random and fixed effects in multilevel logistic regression. Am J Epidemiol. 2005;161:81–8. doi: 10.1093/aje/kwi017. [DOI] [PubMed] [Google Scholar]
- 11.Center for Medicare and Medicaid. [September 21, 2017];Hospital-acquired conditions. 2016 Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalAcqCond/Hospital-Acquired_Conditions.html.
- 12.Agency for Healthcare Research and Quality. [September 21, 2017];2013 annual hospital-acquired condition rate and estimates of cost savings and deaths averted from 2010 to 2013. 2013 Available at: https://www.ahrq.gov/sites/default/files/publications/files/hacrate2013_0.pdf.
- 13.Agency for Healthcare Research and Quality. [September 21, 2017];Partnership for patients. Available at: https://psnet.ahrq.gov/resources/resource/21716.
- 14.Kinlin LM, Kirchner C, Zhang H, Daley J, Fisman DN. Derivation and validation of a clinical prediction rule for nosocomial pneumonia after coronary artery bypass graft surgery. Clin Infect Dis. 2010;50:493–501. doi: 10.1086/649925. [DOI] [PubMed] [Google Scholar]
- 15.Allou N, Bronchard R, Guglielminotti J, et al. Risk factors for postoperative pneumonia after cardiac surgery and development of a preoperative risk score. Crit Care Med. 2014;42:1150–6. doi: 10.1097/CCM.0000000000000143. [DOI] [PubMed] [Google Scholar]
- 16.Strobel RJ, Liang Q, Zhang M, et al. A preoperative risk model for postoperative pneumonia after coronary artery bypass grafting. Ann Thorac Surg. 2016;102:1213–9. doi: 10.1016/j.athoracsur.2016.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donabedian A. The quality of care. How can it be assessed? JAMA. 1988;260:1743–8. doi: 10.1001/jama.260.12.1743. [DOI] [PubMed] [Google Scholar]
- 18.Bryce E, Wong T, Forrester L, et al. Nasal photodisinfection and chlorhexidine wipes decrease surgical site infections: a historical control study and propensity analysis. J Hosp Infect. 2014;88:89–95. doi: 10.1016/j.jhin.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 19.Orringer MB, Marshall B, Chang AC, Lee J, Pickens A, Lau CL. Two thousand transhiatal esophagectomies: changing trends, lessons learned. Ann Surg. 2007;246:363–72. doi: 10.1097/SLA.0b013e31814697f2. discussion 372–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rigotti NA, McKool KM, Shiffman S. Predictors of smoking cessation after coronary artery bypass graft surgery. Results of a randomized trial with 5-year follow-up. Ann Intern Med. 1994;120:287–93. doi: 10.7326/0003-4819-120-4-199402150-00005. [DOI] [PubMed] [Google Scholar]
- 21.Vlietstra RE, Kronmal RA, Oberman A, Frye RL, Killip T., 3rd Effect of cigarette smoking on survival of patients with angiographically documented coronary artery disease. Report from the CASS registry. JAMA. 1986;255:1023–7. [PubMed] [Google Scholar]
- 22.Englesbe MJ, Lussiez AD, Friedman JF, Sullivan JA, Wang SC. Starting a surgical home. Ann Surg. 2015;262:901–3. doi: 10.1097/SLA.0000000000001250. [DOI] [PubMed] [Google Scholar]
- 23.Romagnoli S, Ricci Z. Lung protective ventilation in cardiac surgery. Heart Lung Vessels. 2015;7:5–6. [PMC free article] [PubMed] [Google Scholar]
- 24.Gu WJ, Wang F, Liu JC. Effect of lung-protective ventilation with lower tidal volumes on clinical outcomes among patients undergoing surgery: a meta-analysis of randomized controlled trials. CMAJ. 2015;187:E101–9. doi: 10.1503/cmaj.141005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hudson JK, McDonald BJ, MacDonald JC, Ruel MA, Hudson CC. Impact of subglottic suctioning on the incidence of pneumonia after cardiac surgery: a retrospective observational study. J Cardiothorac Vasc Anesth. 2015;29:59–63. doi: 10.1053/j.jvca.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 26.Perez Granda MJ, Barrio JM, Hortal J, Munoz P, Rincon C, Bouza E. Routine aspiration of subglottic secretions after major heart surgery: impact on the incidence of ventilatorassociated pneumonia. J Hosp Infect. 2013;85:312–5. doi: 10.1016/j.jhin.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Habib RH, Zacharias A, Engoren M, et al. Determinants of prolonged mechanical ventilation after coronary artery bypass grafting. Ann Thorac Surg. 1996;62:1164–71. doi: 10.1016/0003-4975(96)00565-6. [DOI] [PubMed] [Google Scholar]
- 28.Freitas ER, Soares BG, Cardoso JR, Atallah AN. Incentive spirometry for preventing pulmonary complications after coronary artery bypass graft. The Cochrane database of systematic reviews. 2012 doi: 10.1002/14651858.CD004466.pub3. CD004466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saint S, Kowalski CP, Banaszak-Holl J, Forman J, Damschroder L, Krein SL. How active resisters and organizational constipators affect health care-acquired infection prevention efforts. Jt Comm J Qual Patient Saf. 2009;35:239–46. doi: 10.1016/s1553-7250(09)35032-1. [DOI] [PubMed] [Google Scholar]
- 30.Saint S, Kowalski CP, Banaszak-Holl J, Forman J, Damschroder L, Krein SL. The importance of leadership in preventing healthcare-associated infection: Results of a multisite qualitative study. Infect Control Hosp Epidemiol. 2010;31:901–7. doi: 10.1086/655459. [DOI] [PubMed] [Google Scholar]
- 31.Johnson SH, Theurer PF, Bell GF, et al. A statewide quality collaborative for process improvement: internal mammary artery utilization. Ann Thorac Surg. 2010;90:1158–64. doi: 10.1016/j.athoracsur.2010.05.047. discussion 1164. [DOI] [PubMed] [Google Scholar]
- 32.O’Connor GT, Plume SK, Olmstead EM, et al. A regional intervention to improve the hospital mortality associated with coronary artery bypass graft surgery. The northern new england cardiovascular disease study group. JAMA. 1996;275:841–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


