Abstract
The acquired kinase mutation JAK2V617F plays a central role in myeloproliferative neoplasms (MPNs). However, the mechanisms responsible for the malignant hematopoietic stem/progenitor cell (HSPC) expansion seen in patients with MPNs are not fully understood, limiting the effectiveness of current treatment. Endothelial cells (ECs) are an essential component of the hematopoietic niche, and they have been shown to express the JAK2V617F mutation in patients with MPNs. We show that the JAK2V617F-bearing vascular niche promotes the expansion of the JAK2V617F HSPCs in preference to JAK2WT HSPCs, potentially contributing to poor donor cell engraftment and disease relapse following stem cell transplantation. The expression of Chemokine (C-X-C motif) ligand 12 (CXCL12) and stem cell factor (SCF) were upregulated in JAK2V617F-bearing ECs compared to wild-type ECs, potentially accounting for this observation. We further identify that the thrombopoietin (TPO)/MPL signaling pathway is critical for the altered vascular niche function. A better understanding of how the vascular niche contributes to HSPC expansion and MPN development is essential for the design of more effective therapeutic strategies for patients with MPNs.
Introduction
The chronic Philadelphia chromosome (Ph1) negative myeloproliferative neoplasms (MPNs) are clonal stem cell disorders characterized by hematopoietic stem/progenitor cell (HSPC) expansion and overproduction of mature blood cells. The acquired signaling kinase mutation JAK2V617F plays a central role in these disorders, but our understanding of the stem cell expansion that characterizes MPNs remains incomplete, limiting the effectiveness of current treatments. Although the etiology of dysregulated hematopoiesis has been mainly attributed to the molecular alterations within the HSPC compartment, abnormalities of the marrow microenvironment are beginning to be recognized as an important factor in MPN development.1, 2, 3, 4, 5
Endothelial cells (ECs) are an essential component of the hematopoietic niche and most HSPCs reside close to a marrow sinusoid (the ‘perivascular niche’).6, 7, 8, 9 MPNs are characterized by increased marrow angiogenesis compared to normal marrow.10, 11, 12 The JAK2V617F mutation is present in isolated liver or spleen ECs from patients with MPNs.13, 14 The mutation can also be detected in EC progenitors derived from the hematopoietic lineage and, in some reports based on in vitro assays, in the true endothelial colony-forming cells from patients with MPNs.14, 15, 16, 17, 18 Previously, we and others have shown that JAK2V617F-bearing ECs are critical in the development of the bleeding abnormalities in a murine model of JAK2V617F-positive MPNs in which JAK2V617F is expressed in all hematopoietic cells and ECs.19 Our recent study further demonstrated that the JAK2V617F-bearing ECs contribute to the growth advantage of JAK2V617F HSPC over the JAK2WT HSPC in vitro, likely through a critical role of the TPO/MPL signaling axis.20 All of these observations suggest that ECs are involved in the malignant process leading to MPNs.
In the present study, we hypothesized that the JAK2V617F mutation alters the vascular niche to promote MPN HSPC expansion in vivo. To test this hypothesis, we crossed mice that bear a Cre-inducible human JAK2V617F gene (FF1)21 with Tie2-Cre mice22 to express JAK2V617F specifically in all hematopoietic cells and ECs (Tie2/FF1).19, 23 This model has provided us with the ability to study the effect of the JAK2V617F-bearing vascular niche on MPN disease development in vivo. We found that the JAK2V617F-bearing vascular niche contributes to the maintenance and expansion of JAK2V617F HSPCs in preference to JAK2WT HSPCs. These results suggest that the MPN vascular niche can contribute to the poor donor cell engraftment and the high incidence of disease relapse, the two major causes of treatment-related morbidity and mortality associated with the only curative treatment for patients with MPNs, allogeneic stem cell transplantation (SCT).2, 24, 25 Therefore, targeting the MPN vascular niche represents a promising new therapeutic strategy in patients with MPNs.
Materials and methods
Experimental mice
JAK2V617F Flip-Flop (FF1) mice21 was provided by Radek Skoda (University Hospital, Basal, Switzerland), Tie2-Cre mice22 by Mark Ginsberg (University of California, San Diego, CA, USA), and MPL knockout mice (MPL−/−)26 by Warren Alexander (Melbourne, Australia). FF1 mice were crossed with Tie2-Cre mice to express JAK2V617F specifically in hematopoietic cells and ECs (Tie2/FF1 mice). MPL−/− mice were crossed with wild-type (WT) C57BL/6 mice to generate MPL+/− mice. All mice used were crossed onto a C57BL/6 background and bred in a pathogen-free mouse facility at Stony Brook University. CD45.1+ congenic mice (SJL) were purchased from Taconic Inc. (Albany, NY, USA). No randomization or blinding was used to allocate experimental groups. Animal experiments were performed in accordance with the guidelines provided by the Institutional Animal Care and Use Committee.
Additional methods can be found in Supplementary Methods.
Results
The JAK2V617F-bearing vascular niche promotes JAK2V617F-mutant clonal expansion over JAK2WT clones in vivo
Both JAK2WT clones and JAK2V617F mutant clones coexist in most patients with MPNs. Mechanisms responsible for MPN HSPC expansion are not fully understood. In our previous work using an in vitro co-culture system in which Lin−cKit+ HSPCs were cultured on a feeder layer of JAK2WT ECs (isolated from WT mice) or JAK2V617F ECs (isolated from Tie2/FF1 mice), we demonstrated that the JAK2V617F-mutant HSPCs displayed a relative growth advantage over JAK2WT HSPCs when co-cultured on JAK2V617F ECs in vitro.20 This prompted us to ask whether the JAK2V617F-bearing vascular niche contributes to JAK2V617F clonal expansion over JAK2WT clones in vivo.
To address this question, we performed a competitive marrow transplantation experiment. Donor marrow cells from Tie2/FF1 mice (CD45.2) were injected intravenously together with competitor WT marrow cells (CD45.1) into lethally irradiated Tie2/FF1 mice or control mice (CD45.2) (n=5 in each group) (Figure 1a). During a 4-month follow up, Tie2/FF1 recipients displayed a higher peripheral blood CD45.2 (that is, JAK2V617F donor) chimerism than the control mice (P=0.0002), suggesting that the mutant vascular niche in Tie2/FF1 promoted the JAK2V617F clonal maintenance/expansion and/or inhibited WT hematopoiesis (Figure 1b). By 18 weeks post transplant, Tie2/FF1 recipients developed a profound MPN phenotype with both neutrophilia (15.1 vs 3.9 × 109/l, P=0.047) and thrombocytosis (5335 vs 1029 × 109/l, P=0.032) but normal hemoglobin and lymphocyte levels (Figure 1c). Spleens collected from mice 18 weeks after transplantation showed moderate splenomegaly in the Tie2/FF1 recipients compared with the controls (spleen weight 269 mg vs 148 mg, P=0.072)(Figure 1d). Colony formation assays revealed significant increases in burst forming unit-erythroid (BFU-e) (1.8-fold, P=0.026), colony-forming unit granulocyte/macrophage (CFU-GM) (1.7-fold, P=0.006) and total hematopoietic progenitor cells (1.8-fold, P=0.008) (Figure 1e) compared with control mice. Similar results were also obtained with peripheral blood and spleen samples (not shown). There was no difference in total femoral cell count between the Tie2/FF1 recipients and controls.
Figure 1.
JAK2V617F-bearing vascular niche contributes to JAK2V617F clonal expansion in MPN. (a) Competitive marrow transplantation scheme. (b) Following the competitive transplantation experiment of both Tie2/FF1 marrow cells (CD45.2) and competitor wild-type marrow cells (CD45.1), Tie2/FF1 recipients displayed higher peripheral blood CD45.2 (that is, JAK2V617F donor) chimerism than the WT control recipients. (c) By 18 weeks post transplantation, Tie2/FF1 recipients developed a MPN phenotype with significant neutrophilia and thrombocytosis. (d) Spleens collected from mice 18 weeks following transplantation showed moderate splenomegaly in the Tie2/FF1 recipients (black bar) compared with WT recipients (gray bar) (spleen weight 0.269 vs 0.148 g, P=0.073). (e) Tie2/FF1 recipients also exhibited significant increases in total hematopoietic colonies (1.8-fold, P=0.008), BFU-e (1.8-fold, P=0.026), and CFU-GM (1.7-fold, P=0.006) colonies compared with WT recipients. (f) CD45.2+CD150+CD48− (JAK2V617F-mutant) HSPCs were expanded in marrow of Tie2/FF1 recipients compared to WT recipients. In contrast, there was no difference in WT CD45.1+CD150+CD48− cell numbers between the Tie2/FF1 recipients and controls. (g, h) Single colony analysis for JAK2V617F-mutant (g) and JAK2WT donor-derived (h) colonies in Tie2/FF1 recipients and WT recipients.
Since MPNs are characterized by both hematopoietic stem and progenitor cell expansion, we assessed the numbers of a highly enriched stem cell population, CD150+CD48− HSPCs, of which ~20% display long-term repopulating capacity.6 The JAK2V617F-mutant (CD45.2+) CD150+CD48− cell numbers significantly increased in both marrow (FIVEfold, P=0.002, n=5; Figure 1f) and spleen (not shown) of Tie2/FF1 recipients compared with controls. In contrast, there was no significant difference in WT (CD45.1+) CD150+CD48− cell numbers between Tie2/FF1 recipients and controls. Therefore, the JAK2V617F-bearing vascular niche promoted the expansion of JAK2V617F HSPCs but not WT cells.
We next studied whether the JAK2V617F-bearing vascular niche also promoted the JAK2V617F clonal expansion at the progenitor cell level. Marrow cells from Tie2/FF1 and control recipients were cultured on methylcellulose media and individual hematopoietic colonies (both erythoid and myeloid) were plucked from each sample for genotyping. We found that there were significantly more JAK2V617F-derived colony forming cells in Tie2/FF1 recipients than in controls (2.9-fold, P=0.025; Figure 1g). In addition, WT donor derived colonies were present in three of five control recipients while none was detected in Tie2/FF1 recipients (Figure 1h). These findings indicate that the JAK2V617F-bearing vascular niche promoted the expansion of JAK2V617F progenitors, probably at the expense of suppressing WT hematopoiesis.
In contrast, the control mice who received donor cells from both Tie2/FF1 mice and WT mice had mostly normal blood cell counts during the 4-month follow up (Figure 1c). Evaluation of the HSPC compartment did not reveal any significant difference between CD45.2+CD150+CD48− (JAK2V617F-mutant) and CD45.1+CD150+CD48− (wild-type) cell frequency in the control mice (Figure 1f). These observations suggest that the JAK2V617F-mutant HSPCs alone are either not sufficient to cause a MPN, or need a longer period of time to develop the disease compared to when both mutant HSPCs and an altered microenvironment (for example, JAK2V617F-bearing ECs) are present.
The JAK2V617F mutation alters vascular niche function to contribute to HSPC expansion
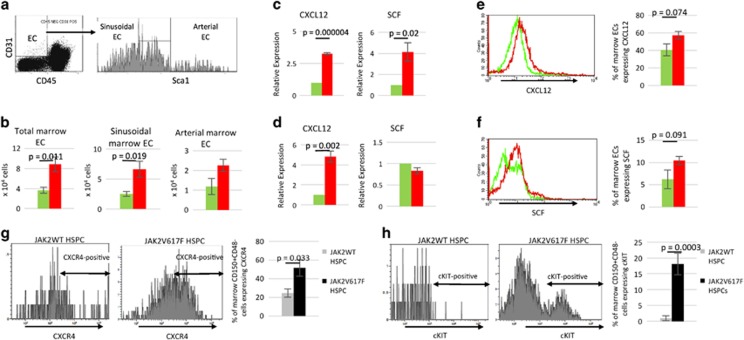
Having demonstrated that the JAK2V617F-bearing vascular niche promotes neoplastic hematopoiesis in MPN, we then investigated how the JAK2V617F mutation affects vascular niche function. We found that total marrow EC (CD45−CD31+), sinusoidal marrow EC (CD45−CD31+Sca1−)27 and arterial marrow EC (CD45−CD31+Sca1+)27 cell numbers were increased in the Tie2/FF1 recipients (n=5) compared to control mice (n=5) following competitive transplantation (Figures 2a and b). This is consistent with our previous report that JAK2V617F-bearing ECs proliferate to a greater extent than JAK2WT ECs and display significantly increased angiogenesis in vitro compared to JAK2WT ECs,20 a finding that also characterizes the marrow of patients with MPNs.10, 11, 12
Figure 2.
The JAK2V617F mutation alters vascular niche function. (a) Representative flow cytometry density and histogram plots for marrow ECs. (b) Total marrow EC, sinusoidal marrow EC, and arterial marrow EC number in Tie2/FF1 recipients (red bar, n=5) and WT recipients (green bar, n=5). (c, d) The expression levels of CXCL12 and SCF in pooled arterial (c) and sinusoidal (d) marrow ECs of Tie2/FF1 recipients (red bar, n=5) and WT recipients (green bar, n=5) were measured using real-time qPCR. Gene expression in Tie2/FF1 marrow ECs is shown as the fold-change compared with the WT marrow EC expression which was set as ‘1’. (e, f) Representative histogram plots and flow cytometry quantitative analysis of intracellular CXCL12 (e) and membranal SCF (f) expression in marrow ECs of Tie2/FF1 recipients (red) and WT recipients (green) (n=3-5). (g, h) Representative histogram plots and flow cytometry quantitative analysis of membranal CXCR4 (g) and cKIT (h) expression in JAK2WT HSPCs (gray bar) and JAK2V617F HSPCs (black bar) in the Tie2/FF1 recipients (n=4).
To begin to understand the EC signals responsible for JAK2V617F HSPC expansion and MPN pathogenesis, we measured the expression levels of CXCL12 and SCF, two essential niche factors for HSPC maintenance,28, 29, 30 in marrow ECs of Tie2/FF1 mice (n=5) and control mice (n=5) from the competitive transplantation experiment. qPCR analysis confirmed that there was upregulation of CXCL12 in both arterial (3.3-fold, P=0.000004) and sinusoidal (4.8-fold, P=0.002) marrow ECs while SCF was upregulated in arterial marrow ECs (4.1-fold, P=0.02) in Tie2/FF1 mice compared to control mice (Figures 2c and d). Flow cytometry quantitative analysis showed that the proportion of marrow ECs expressing CXCL12 (41% increase, P=0.074) or SCF (68% increase, P=0.091) was increased in the Tie2/FF1 mice as compared to control mice (Figures 2e and f). CXCL12 and SCF have been shown to promote HSPC survival and protect HSPCs from irradiation-induced cell death.31, 32, 33 In chronic myelogenous leukemia, another MPN (Ph1 positive) driven by deregulated tyrosine kinase activity, SCF induces selective expansion of the leukemic HSPCs over normal HSPCs.34, 35 Therefore, increased CXCL12 and SCF levels in the JAK2V617F-bearing marrow ECs could each or collectively contribute to the HSPC expansion phenotype we have observed in the Tie2/FF1 recipients.
To understand how the altered niche factors contribute to JAK2V617F clonal expansion in the Tie2/FF1 recipients, we measured the expression of the CXCL12 receptor CXCR4 and the SCF receptor cKIT in both JAK2WT (CD45.1) CD150+CD48− HSPCs and JAK2V617F (CD45.2) CD150+CD48− HSPCs from the Tie2/FF1 recipients. We found that the proportion of cells expressing CXCR4 (2.1-fold, P=0.033) or cKIT (19-fold, P=0.0003) was significantly increased in the JAK2V617F HSPCs compared to JAK2WT HSPCs (Figures 2g and h). Therefore, the increased CXCL12 and SCF levels in the JAK2V617F-bearing ECs could mediate the clonal expansion of JAK2V617F HSPCs, via the upregulated CXCR4 and cKIT receptors, over JAK2WT HSPCs in the Tie2/FF1 recipients.
The EC MPL receptor contributes to the maintenance/expansion of the JAK2V617F HSPC over JAK2WT HSPCs
Thrombopoietin (TPO) and its receptor, the proto-oncogene MPL, are key regulators of HSPC activity.36, 37 Previously, we and others have shown that MPL is essential for the development of an increased neoplastic stem cell pool in MPNs.38, 39 MPL is expressed on several types of ECs.40, 41, 42 Whether the EC MPL receptor could affect vascular niche function, and contribute to the critical role of TPO/MPL signaling in HSPC maintenance, is not known.
Consistent with our previous report that MPL expression was increased in JAK2V617F-bearing lung ECs compared to JAK2WT lung ECs,20 MPL expression was also significantly increased in both arterial marrow ECs (2.9-fold, P=0.038) and sinusoidal marrow ECs (1.7-fold, P=0.004) of the Tie2/FF1 recipients compared to control mice (Figure 3a). Using an in vitro competitive growth assay where both JAK2WT HSPCs (CD45.1) and JAK2V617F HSPCs (CD45.2) were cultured together in the presence of conditioned medium collected from either WT or MPL knockout lung ECs (isolated from WT or MPL−/− mice), we have previously shown that the EC MPL receptor is important for the maintenance/expansion of the JAK2V617F clone over the JAK2WT clone in vitro.20 This led us to hypothesize that certain secreted factors from the vascular ECs contribute to the JAK2V617F clonal expansion in MPNs, and that TPO/MPL signaling is critical for this vascular niche function. Therefore, we measured the expression levels of CXCL12 and SCF in marrow ECs isolated from WT mice, MPL heterozygous mice (MPL+/−) and MPL knockout mice (MPL−/−). We confirmed that there was significant downregulation of CXCL12 and SCF in MPL−/− marrow ECs compared with WT cells, in a dose-dependent fashion (Figure 3b). These results suggest that altered TPO/MPL signaling can affect vascular niche function and contribute to HSPC expansion in the Tie2/FF1 recipient mice.
Figure 3.
Altered EC TPO/MPL signaling affects the vascular niche function in Tie2/FF1 mice. (a) MPL expression was significantly increased in both arterial marrow ECs and sinusoidal marrow ECs of the Tie2/FF1 recipients compared to control mice. Gene expression is shown as the fold-change compared with the control marrow EC expression which was set as ‘1’. (b) The expression levels of CXCL12 and SCF in WT marrow ECs, MPL+/− marrow ECs and MPL−/− marrow ECs were measured using real-time qPCR. Gene expression in MPL+/− marrow ECs, and MPL−/− marrow ECs is shown as the relative fold-change compared with WT EC expression which was set as ‘1’.
The JAK2V617F mutation alters the megakaryocyte–vascular niche interactions to promote HSPC expansion
Megakaryocytes (MK) are rare polyploid marrow cells that give rise to blood platelets. Very recent evidence has implicated MKs in regulating HSPC activity.43, 44, 45 MKs are often located adjacent to marrow sinusoids, a ‘geography’ required for the cells to issue platelets directly into the sinusoidal vascular lumen.46 Considering that most HSPCs reside close to a marrow sinusoid, the interactions between MKs and ECs in the vascular niche are positioned to play an important role in modulating HSPC function.
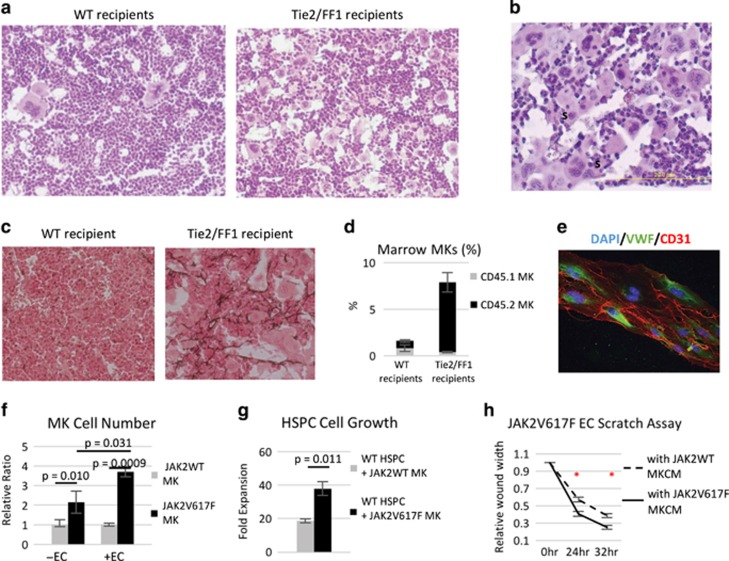
Consistent with the thrombocytosis seen in Tie2/FF1 recipients, histological analysis of marrow hematoxylin/eosin sections revealed markedly increased numbers of MKs in these mice compared to controls. Clusters of MKs were preferentially located near sinusoid vessels in Tie2/FF1 recipients (Figures 4a and b). In addition, reticulin fibrosis was present in the marrow of Tie2/FF1 recipients, but absent in control recipients (Figure 4c). Since MKs play an important role in the pathogenesis of marrow fibrosis, these findings suggest that JAK2V617F MKs may contribute to the development of a full-blown MPN with marrow fibrosis in the Tie2/FF1 recipients.
Figure 4.
The JAK2V617F mutation alters the MK–vascular niche interactions to promote HSPC expansion. (a) There were markedly increased numbers of MKs in Tie2/FF1 recipient mice (right) compared with WT recipients (left). (b) Clusters of MKs were preferentially located near sinusoids (S). (c) Reticulin staining of marrow demonstrates fibrosis in 3/3 Tie2/FF1 recipients, but not in the three examined WT recipients. (d) CD45.2+ (JAK2V617F-mutant) MKs (black bar) increased significantly in the Tie2/FF1 mice (n=5) compared to controls (n=5), while there was no significant difference of the CD45.1+ (wild-type) MKs (gray bar) between the two groups. (e) The fluorescence image of ECs. DAPI (blue), Von Willebrand Factor (VWF) (green), CD31 (red). (f) JAK2V617F MKs gained a greater growth advantage compared to JAK2WT MKs when co-cultured on a feeder layer of JAK2V617F spleen ECs than when cultured in SFEM alone. JAK2V617F MK cell proliferation was shown as the relative ratio compared to JAK2WT MKs cultured under the same conditions. Data are from two independent experiments with triplicates in each experiment. (g) JAK2V617F MKs promoted CD150+CD48− HSPC proliferation more than JAK2WT MKs did in an in vitro direct co-culture experiment. Cell proliferation was shown as fold of expansion which is the ratio of the final cell count to starting cell count. Data are from three independent experiments with triplicates in each experiment. (h) A scratch assay was performed in JAK2V617F spleen ECs. JAK2V617F MKCM significantly stimulated JAK2V617F EC migration compared to that of WT MKCM. The distances from one side of the scratch wound to the other side were measured using ImageJ software (National Institute of Health, Bethesda, MD, USA) at six different locations for each culture condition. The distance of wound closure at time 24 and 48 h was compared with the distance at time 0 h which was set as 1. The results were expressed as the mean±s.e.m. (n=6). Data are from one of two independent experiments that gave similar results.
Quantitative evaluation revealed that marrow JAK2V617F MK (CD45.2+CD41+) cell frequency was significantly increased in the Tie2/FF1 recipients compared to controls (9.6-fold, P=0.0002, n=5) although both groups were transplanted with 50% JAK2V617F-mutant marrow cells (Figure 4d). In contrast, there was no significant difference of the WT MKs (CD45.1+CD41+) between the two groups. To further test whether the JAK2V617F-bearing vascular niche stimulates JAK2V617F MK expansion directly, we isolated JAK2V617F-bearing ECs from the Tie2/FF1 mice and performed a MK-EC co-culture experiment. We found that JAK2V617F MKs gained a greater growth advantage compared to JAK2WT MKs when co-cultured on JAK2V617F ECs than when cultured in serum-free expansion medium (SFEM) alone (3.9-fold vs 2.2-fold, P=0.031), suggesting that the JAK2V617F-bearing ECs can directly stimulate JAK2V617F MK expansion (Figures 4e and f).
We then examined whether JAK2V617F MKs stimulate HSPC expansion more than JAK2WT MKs do using an in vitro co-culture experiment where WT CD150+CD48− HSPCs (CD45.1) were cultured together with JAK2WT or JAK2V617F MKs (CD45.2). At the end of the 12-day culture, there were more CD45.1 cells when co-cultured together with JAK2V617F MKs than when co-cultured together with JAK2WT MKs (twofold, P=0.011; Figure 4g).
Finally, after demonstrating that the JAK2V617F-bearing ECs can expand the JAK2V617F-bearing MKs which in turn contributes to HSPC expansion, we tested whether the JAK2V617F MKs could affect JAK2V617F EC function in vitro. A scratch assay was performed to measure EC migration in vitro and we found that JAK2V617F MK-conditioned medium (MKCM) significantly stimulated JAK2V617F EC migration compared to that of WT MKCM. (Figure 4h) Taken together, these data indicate that the JAK2V617F mutation alters the MK–endothelial interactions in the vascular niche to promote HSPC expansion in the Tie2/FF1 recipients.
Discussion
Although previous studies have reported that a diseased microenvironment or perturbed interactions between the hematopoietic cells and the niche could cause myeloproliferation, mutations tested in those studies were not common in patients with MPNs and the responsible niche cells were not specified.1, 5 Here, by crossing the previously described human JAK2V617F knock-in mice (FF1)21 with the Tie2-Cre mice,22 we have been able to highlight the importance of the JAK2V617F-bearing vascular niche in the abnormal hematopoiesis of MPNs.
In our competitive marrow transplantation experiments where both JAK2V617F marrow cells (CD45.2) and WT marrow cells (CD45.1) were injected together into lethally irradiated recipient mice, the Tie2/FF1 recipients (with the JAK2V617F-bearing vascular niche) developed a profound MPN phenotype, while the WT recipients did not. While WT donor-derived progenitor cells were still present in three of five control recipients 18 weeks following transplantation, none was detected in the Tie2/FF1 recipients (n=5) (Figure 1). Two conclusions can be drawn from these data to guide future investigation. First, these data provide clear evidence that the JAK2V617F-bearing vascular niche promotes JAK2V617F clonal expansion while inhibiting WT hematopoiesis. These data are consistent with other reports that the marrow microenvironment of myeloid malignancies are altered to impair normal hematopoiesis, while favoring malignant stem cell expansion.2, 47 Second, these observations indicate that in the competitive transplantation environment, where both JAK2V617F marrow cells and JAK2WT marrow cells were transplanted together, the JAK2V617F-mutant HSPCs alone are either insufficient to develop a MPN phenotype in the absence of additional disease-promoting mechanisms (for example, V617F bearing niche) or require a longer period of time to develop the disease phenotype in WT environment (that is, WT recipient) than in mutant environment (that is, Tie2/FF1 recipients). This is surprising and appears in conflict with prior reports that the JAK2V617F-positive MPN phenotype is transplantable and usually develops as early as 4 weeks after transplanting 100% JAK2V617F-positive marrow cells into WT recipients.19, 48, 49, 50, 51 It is possible that, in our competitive transplantation experiment where 50% JAK2V617F marrow cells and 50% JAK2WT marrow cells were transplanted together, the JAK2V617F-mutant HSPCs require a longer period of time to develop the disease phenotype in WT recipients. Another possibility is that the JAK2V617F-mutant HSPCs have little selective advantage over WT HSPCs when transplanted into mice that bear a normal niche.49, 51, 52, 53 This may explain why we and others51 did not observe any MPN phenotype with JAK2V617F-mutant HSPCs in the competitive transplant setting. This is consistent with our previous report that there was no significant difference between JAK2WT and JAK2V617F HSPC proliferation when co-cultured with JAK2WT EC.20 It is also supported by the clinical observation that in some patients with MPNs, there is coexistence of the mutant clone and the WT clone with no change in the mutant/WT cell ratio over long term follow up.54, 55, 56 In essence, crosstalk between the altered HSPCs and the altered microenvironment is likely required to provide the ‘selective pressure’ for the mutant HSPCs to outcompete WT HSPCs in the development of a MPN.
CXCL12 and SCF, two important niche factors for HSPC maintenance,28, 29, 30 are upregulated in JAK2V617F-bearing ECs compared to WT ECs. In addition, expression of the CXCL12 receptor CXCR4 and the SCF receptor c-KIT is upregulated in JAK2V617F marrow HSPCs compared to JAK2WT marrow HSPCs in our competitive marrow transplantation experiment (Figure 2). Therefore, the increased CXCL12 and SCF levels in the JAK2V617F-bearing vascular niche could contribute to the clonal expansion of JAK2V617F HSPC via its upregulated CXCR4 receptor. Although this appears contradictory to other reports that CXCR4 is mostly down regulated in spleen and peripheral blood HSPCs from patients of MPNs,57, 58, 59 it suggests that there is increased CXCL12/CXCR4 signaling in MPN HSPCs,60 which would favor their maintenance within the bone marrow microenvironment and extramedullar sites. Further studies (for example, conditional deletion of CXCL12 and/or SCF in JAK2V617F-bearing ECs) are required to understand how these vascular niche factors contribute to uncontrolled HSPC expansion leading to MPNs. In this study, we also found that MPL expression is significantly increased in the JAK2V617F-bearing marrow ECs and knock-out of MPL in ECs significantly decreases the levels of CXCL12 and SCF. These results suggest that altered TPO/MPL signaling can affect vascular niche function and contribute to HSPC expansion in the Tie2/FF1 recipient mice. Ablating MPL in the JAK2V617F-bearing ECs will be required to determine the roles of TPO/MPL signaling in the hematopoietic vascular niche in MPNs.
Our studies also found that the JAK2V617F mutation alters MK–endothelial interactions in the hematopoietic vascular niche. Previously we found that JAK2V617F MKs stimulate EC angiogenesis in vitro and cause a murine myeloproliferative syndrome with HSPC expansion and increased sinusoidal vascular density in vivo.23 In the current work, we demonstrated that the JAK2V617F-bearing ECs promote JAK2V617F-mutant MK expansion over WT MKs, which in turn contributes to HSPC expansion. In contrast to non-hematopoietic niche cells (for example, ECs, mesenchymal stromal cells), niche MKs provide direct feedback to their precursor HSPCs, many of which are located directly adjacent to MKs in vivo,43, 44 and therefore may play important roles in malignant HSPC clonal expansion during neoplastic hematopoiesis. Since both MKs and ECs represent important sources of bioactive hematopoietic factors and express many adhesion molecules that are active in hematopoiesis, systemic analysis of MK and EC proteins using either quantitative proteomics or antibody-based arrays would be required to further investigate the interaction between MKs and ECs in both normal and neoplastic hematopoiesis.
Although the JAK2V617F mutation has only been reported in the liver and spleen ECs from patients with MPNs,13, 14 it very likely also exists in their marrow ECs considering that liver, spleen, and marrow are all hematopoietic organs during embryonic development and/or throughout adulthood. The stem cell compartment in MPN is heterogeneous with the presence of both JAK2 WT clones and the JAK2V617F mutant clones in most MPN patients. We hypothesize that the vascular niche in MPN patients is also heterogeneous with the co-existence of both normal and mutant ECs. Since the JAK2V617F mutation is present in all HSPCs and ECs in the Tie2/FF1 mice, our murine model may not recapitulate the clonality features that are present in patients with MPNs. Nonetheless, our study has demonstrated that the JAK2V617F-bearing vascular niche can contribute to the expansion of JAK2V617F HSPCs in preference to JAK2WT HSPCs, which provides a mechanism for the poor donor cell engraftment and the high incidence of disease relapse in patients, the two major causes of treatment-related morbidity and mortality associated with the only curative treatment for patients with MPNs, allogeneic SCT. Therefore, targeting the MPN vascular niche could provide a promising new therapeutic strategy for patients with MPNs. Further investigation would be required to model the in vivo condition where both normal and mutant ECs are likely co-exist.
Acknowledgments
We thank Dr Wei Hou (Stony Brook University, NY) for his consultation on statistical analysis and Dr Yu Zhang (Peking Union Medical College, China) for her assistance with some of the flow cytometry work. This research was supported by the Veterans Affairs award IK2BX001559 (HZ) and National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK049855 (KK).
Author contributions
HZ designed and performed experiments, analyzed data, and wrote the manuscript; CL performed experiments, analyzed data and reviewed the manuscript; YS contributed to part of the mouse work and single colony genotype; KK designed experiments, analyzed data and edited the manuscript.
Footnotes
Supplementary Information accompanies this paper on the Leukemia website (http://www.nature.com/leu)
The authors declare no conflict of interest.
Supplementary Material
References
- Walkley CR, Olsen GH, Dworkin S, Fabb SA, Swann J, McArthur GA et al. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell 2007; 129: 1097–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers K, Pietras EM, Reynaud D, Flach J, Binnewies M, Garg T et al. Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. Cell Stem Cell 2013; 13: 285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arranz L, Sanchez-Aguilera A, Martin-Perez D, Isern J, Langa X, Tzankov A et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature 2014; 512: 78–81. [DOI] [PubMed] [Google Scholar]
- Mager LF, Riether C, Schurch CM, Banz Y, Wasmer MH, Stuber R et al. IL-33 signaling contributes to the pathogenesis of myeloproliferative neoplasms. J Clin Investig 2015; 125: 2579–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkley CR, Shea JM, Sims NA, Purton LE, Orkin SH. Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell 2007; 129: 1081–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 2005; 121: 1109–1121. [DOI] [PubMed] [Google Scholar]
- Sipkins DA, Wei X, Wu JW, Runnels JM, Cote D, Means TK et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature 2005; 435: 969–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acar M, Kocherlakota KS, Murphy MM, Peyer JG, Oguro H, Inra CN et al. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature 2015; 526: 126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inra CN, Zhou BO, Acar M, Murphy MM, Richardson J, Zhao Z et al. A perisinusoidal niche for extramedullary haematopoiesis in the spleen. Nature 2015; 527: 466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medinger M, Skoda R, Gratwohl A, Theocharides A, Buser A, Heim D et al. Angiogenesis and vascular endothelial growth factor-/receptor expression in myeloproliferative neoplasms: correlation with clinical parameters and JAK2-V617F mutational status. Br J Haematol 2009; 146: 150–157. [DOI] [PubMed] [Google Scholar]
- Boveri E, Passamonti F, Rumi E, Pietra D, Elena C, Arcaini L et al. Bone marrow microvessel density in chronic myeloproliferative disorders: a study of 115 patients with clinicopathological and molecular correlations. Br J Haematol 2008; 140: 162–168. [DOI] [PubMed] [Google Scholar]
- Gianelli U, Vener C, Raviele PR, Savi F, Somalvico F, Calori R et al. VEGF expression correlates with microvessel density in Philadelphia chromosome-negative chronic myeloproliferative disorders. Am J Clin Pathol 2007; 128: 966–973. [DOI] [PubMed] [Google Scholar]
- Sozer S, Fiel MI, Schiano T, Xu M, Mascarenhas J, Hoffman R. The presence of JAK2V617F mutation in the liver endothelial cells of patients with Budd-Chiari syndrome. Blood 2009; 113: 5246–5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosti V, Villani L, Riboni R, Poletto V, Bonetti E, Tozzi L et al. Spleen endothelial cells from patients with myelofibrosis harbor the JAK2V617F mutation. Blood 2013; 121: 360–368. [DOI] [PubMed] [Google Scholar]
- Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 2007; 109: 1801–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teofili L, Martini M, Iachininoto MG, Capodimonti S, Nuzzolo ER, Torti L et al. Endothelial progenitor cells are clonal and exhibit the JAK2(V617F) mutation in a subset of thrombotic patients with Ph-negative myeloproliferative neoplasms. Blood 2011; 117: 2700–2707. [DOI] [PubMed] [Google Scholar]
- Sozer S, Ishii T, Fiel MI, Wang J, Wang X, Zhang W et al. Human CD34+ cells are capable of generating normal and JAK2V617F positive endothelial like cells in vivo. Blood Cells, Mol Dis 2009; 43: 304–312. [DOI] [PubMed] [Google Scholar]
- Piaggio G, Rosti V, Corselli M, Bertolotti F, Bergamaschi G, Pozzi S et al. Endothelial colony-forming cells from patients with chronic myeloproliferative disorders lack the disease-specific molecular clonality marker. Blood 2009; 114: 3127–3130. [DOI] [PubMed] [Google Scholar]
- Etheridge SL, Roh ME, Cosgrove ME, Sangkhae V, Fox NE, Chen J et al. JAK2V617F-positive endothelial cells contribute to clotting abnormalities in myeloproliferative neoplasms. Proc Natl Acad Sci USA 2014; 111: 2295–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Kaushansky K, Zhan H. JAK2V617F-mutant vascular niche contributes to JAK2V617F clonal expansion in myeloproliferative neoplasms. Blood Cells Mol Dis 2016; 62: 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedt R, Hao-Shen H, Sobas MA, Looser R, Dirnhofer S, Schwaller J et al. Ratio of mutant JAK2-V617F to wild-type Jak2 determines the MPD phenotypes in transgenic mice. Blood 2008; 111: 3931–3940. [DOI] [PubMed] [Google Scholar]
- Constien R, Forde A, Liliensiek B, Grone HJ, Nawroth P, Hammerling G et al. Characterization of a novel EGFP reporter mouse to monitor Cre recombination as demonstrated by a Tie2 Cre mouse line. Genesis 2001; 30: 36–44. [DOI] [PubMed] [Google Scholar]
- Zhan H, Ma Y, Lin CH, Kaushansky K. JAK2V617F-mutant megakaryocytes contribute to hematopoietic stem/progenitor cell expansion in a model of murine myeloproliferation. Leukemia 2016; 30: 2332–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroger N, Holler E, Kobbe G, Bornhauser M, Schwerdtfeger R, Baurmann H et al. Allogeneic stem cell transplantation after reduced-intensity conditioning in patients with myelofibrosis: a prospective, multicenter study of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Blood 2009; 114: 5264–5270. [DOI] [PubMed] [Google Scholar]
- Rondelli D, Goldberg JD, Isola L, Price LS, Shore TB, Boyer M et al. MPD-RC 101 prospective study of reduced-intensity allogeneic hematopoietic stem cell transplantation in patients with myelofibrosis. Blood 2014; 124: 1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander WS, Roberts AW, Nicola NA, Li R, Metcalf D. Deficiencies in progenitor cells of multiple hematopoietic lineages and defective megakaryocytopoiesis in mice lacking the thrombopoietic receptor c-Mpl. Blood 1996; 87: 2162–2170. [PubMed] [Google Scholar]
- Itkin T, Gur-Cohen S, Spencer JA, Schajnovitz A, Ramasamy SK, Kusumbe AP et al. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature 2016; 532: 323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 2012; 481: 457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 2013; 495: 231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 2013; 495: 227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer HE, Cooper S, Kohli L, Hangoc G, Lee Y, Mantel C et al. Transgenic expression of stromal cell-derived factor-1/CXC chemokine ligand 12 enhances myeloid progenitor cell survival/antiapoptosis in vitro in response to growth factor withdrawal and enhances myelopoiesis in vivo. J Immunol 2003; 170: 421–429. [DOI] [PubMed] [Google Scholar]
- Lataillade JJ, Clay D, Bourin P, Herodin F, Dupuy C, Jasmin C et al. Stromal cell-derived factor 1 regulates primitive hematopoiesis by suppressing apoptosis and by promoting G(0)/G(1) transition in CD34(+) cells: evidence for an autocrine/paracrine mechanism. Blood 2002; 99: 1117–1129. [DOI] [PubMed] [Google Scholar]
- Lee Y, Gotoh A, Kwon HJ, You M, Kohli L, Mantel C et al. Enhancement of intracellular signaling associated with hematopoietic progenitor cell survival in response to SDF-1/CXCL12 in synergy with other cytokines. Blood 2002; 99: 4307–4317. [DOI] [PubMed] [Google Scholar]
- Moore S, Haylock DN, Levesque JP, McDiarmid LA, Samels LM, To LB et al. Stem cell factor as a single agent induces selective proliferation of the Philadelphia chromosome positive fraction of chronic myeloid leukemia CD34(+) cells. Blood 1998; 92: 2461–2470. [PubMed] [Google Scholar]
- Agarwal R, Doren S, Hicks B, Dunbar CE. Long-term culture of chronic myelogenous leukemia marrow cells on stem cell factor-deficient stroma favors benign progenitors. Blood 1995; 85: 1306–1312. [PubMed] [Google Scholar]
- Yoshihara H, Arai F, Hosokawa K, Hagiwara T, Takubo K, Nakamura Y et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell 2007; 1: 685–697. [DOI] [PubMed] [Google Scholar]
- Qian H, Buza-Vidas N, Hyland CD, Jensen CT, Antonchuk J, Mansson R et al. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell 2007; 1: 671–684. [DOI] [PubMed] [Google Scholar]
- Sangkhae V, Etheridge SL, Kaushansky K, Hitchcock IS. The thrombopoietin receptor, MPL, is critical for development of a JAK2V617F-induced myeloproliferative neoplasm. Blood 2014; 124: 3956–3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty C, Pecquet C, Nivarthi H, El-Khoury M, Chachoua I, Tulliez M et al. Calreticulin mutants in mice induce an MPL-dependent thrombocytosis with frequent progression to myelofibrosis. Blood 2016; 127: 1317–1324. [DOI] [PubMed] [Google Scholar]
- Cardier JE, Dempsey J. Thrombopoietin and its receptor, c-mpl, are constitutively expressed by mouse liver endothelial cells: evidence of thrombopoietin as a growth factor for liver endothelial cells. Blood 1998; 91: 923–929. [PubMed] [Google Scholar]
- Brizzi MF, Battaglia E, Montrucchio G, Dentelli P, Del Sorbo L, Garbarino G et al. Thrombopoietin stimulates endothelial cell motility and neoangiogenesis by a platelet-activating factor-dependent mechanism. Circ Res 1999; 84: 785–796. [DOI] [PubMed] [Google Scholar]
- Geddis AE, Fox NE, Kaushansky K. The Mpl receptor expressed on endothelial cells does not contribute significantly to the regulation of circulating thrombopoietin levels. Exp Hematol 2006; 34: 82–86. [DOI] [PubMed] [Google Scholar]
- Zhao M, Perry JM, Marshall H, Venkatraman A, Qian P, He XC et al. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat Med 2014; 20: 1321–1326. [DOI] [PubMed] [Google Scholar]
- Bruns I, Lucas D, Pinho S, Ahmed J, Lambert MP, Kunisaki Y et al. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat Med 2014; 20: 1315–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura-Ishizu A, Takubo K, Kobayashi H, Suzuki-Inoue K, Suda T. CLEC-2 in megakaryocytes is critical for maintenance of hematopoietic stem cells in the bone marrow. J Exp Med 2015; 212: 2133–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junt T, Schulze H, Chen Z, Massberg S, Goerge T, Krueger A et al. Dynamic visualization of thrombopoiesis within bone marrow. Science 2007; 317: 1767–1770. [DOI] [PubMed] [Google Scholar]
- Zhang B, Ho YW, Huang Q, Maeda T, Lin A, Lee SU et al. Altered microenvironmental regulation of leukemic and normal stem cells in chronic myelogenous leukemia. Cancer Cell 2012; 21: 577–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig G, Mercher T, Okabe R, Levine RL, Lee BH, Gilliland DG. Expression of Jak2V617F causes a polycythemia vera-like disease with associated myelofibrosis in a murine bone marrow transplant model. Blood 2006; 107: 4274–4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullally A, Lane SW, Ball B, Megerdichian C, Okabe R, Al-Shahrour F et al. Physiological Jak2V617F expression causes a lethal myeloproliferative neoplasm with differential effects on hematopoietic stem and progenitor cells. Cancer Cell 2010; 17: 584–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akada H, Yan D, Zou H, Fiering S, Hutchison RE, Mohi MG. Conditional expression of heterozygous or homozygous Jak2V617F from its endogenous promoter induces a polycythemia vera-like disease. Blood 2010; 115: 3589–3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Spensberger D, Ahn JS, Anand S, Beer PA, Ghevaert C et al. JAK2 V617F impairs hematopoietic stem cell function in a conditional knock-in mouse model of JAK2 V617F-positive essential thrombocythemia. Blood 2010; 116: 1528–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent DG, Li J, Tanna H, Fink J, Kirschner K, Pask DC et al. Self-renewal of single mouse hematopoietic stem cells is reduced by JAK2V617F without compromising progenitor cell expansion. PLoS Biol 2013; 11: e1001576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Kent DG, Godfrey AL, Manning H, Nangalia J, Aziz A et al. JAK2V617F homozygosity drives a phenotypic switch in myeloproliferative neoplasms, but is insufficient to sustain disease. Blood 2014; 123: 3139–3151. [DOI] [PubMed] [Google Scholar]
- Gale RE, Allen AJ, Nash MJ, Linch DC. Long-term serial analysis of X-chromosome inactivation patterns and JAK2 V617F mutant levels in patients with essential thrombocythemia show that minor mutant-positive clones can remain stable for many years. Blood 2007; 109: 1241–1243. [DOI] [PubMed] [Google Scholar]
- Lambert JR, Gale RE, Linch DC. The production of JAK2 wild-type platelets is not downregulated in patients with JAK2 V617F mutant-positive essential thrombocythaemia. Br J Haematol 2009; 145: 128–130. [DOI] [PubMed] [Google Scholar]
- Stein BL, Williams DM, Wang NY, Rogers O, Isaacs MA, Pemmaraju N et al. Sex differences in the JAK2 V617F allele burden in chronic myeloproliferative disorders. Haematologica 2010; 95: 1090–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosti V, Massa M, Vannucchi AM, Bergamaschi G, Campanelli R, Pecci A et al. The expression of CXCR4 is down-regulated on the CD34+ cells of patients with myelofibrosis with myeloid metaplasia. Blood Cells Mol Dis 2007; 38: 280–286. [DOI] [PubMed] [Google Scholar]
- Guglielmelli P, Zini R, Bogani C, Salati S, Pancrazzi A, Bianchi E et al. Molecular profiling of CD34+ cells in idiopathic myelofibrosis identifies a set of disease-associated genes and reveals the clinical significance of Wilms' tumor gene 1 (WT1). Stem Cells 2007; 25: 165–173. [DOI] [PubMed] [Google Scholar]
- Wang X, Cho SY, Hu CS, Chen D, Roboz J, Hoffman R. C-X-C motif chemokine 12 influences the development of extramedullary hematopoiesis in the spleens of myelofibrosis patients. Exp Hematol 2015; 43: 100–109 e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelouahab H, Zhang Y, Wittner M, Oishi S, Fujii N, Besancenot R et al. CXCL12/CXCR4 pathway is activated by oncogenic JAK2 in a PI3K-dependent manner. Oncotarget 2016; epub ahead of print 22 July 2016; doi:10.18632/oncotarget.10789. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.