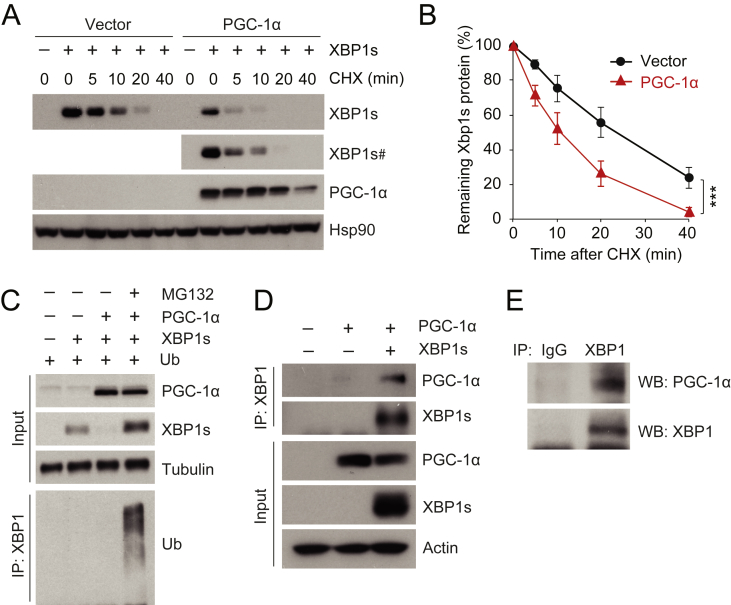
Figure 2.
PGC-1α physically interacts with XBP1s and promotes ubiquitin-mediated XBP1s protein degradation. (A, B) HEK293 cells, transfected with a plasmid expressing XBP1s plus empty vector or a plasmid expressing PGC-1α, were treated with cycloheximide (CHX, 50 μg/ml) for the indicated time. (A) Levels of XBP1s, PGC-1α, and Hsp90 protein levels. # indicates longer exposed film. (B) The quantified ratio of XBP1s to Hsp90 protein in A before and after treatment with CHX. (C) XBP1s, PGC-1α, tubulin, and ubiquitinated XBP1s protein levels. HEK293 cells expressing ubiquitin and XBP1s with or without PGC-1α were treated with DMSO or MG132 (10 μM) for 1 h. Ubiquitinated XBP1s was analyzed from immunoprecipitates pulled down with antibody specific to XBP1 (anti-XBP1), by immunoblotting with anti-ubiquitin. (D) Co-immunoprecipitation of XBP1s and PGC-1α. HEK293 cells were transfected with plasmid expressing PGC-1α, with or without the plasmid that encodes XBP1s. Cells were lysed and immunoprecipitated with anti-XBP1. (E) Co-immunoprecipitation of endogenous XBP1s and PGC-1α. Eight week-old male mice were fasted for 24 h, then refed for 90 min. Liver protein lysates were immunoprecipitated with either rabbit IgG or anti-XBP1, and co-immunoprecipitates were analyzed by western blotting. Each experiment was independently reproduced at least three times. Values are means ± s.e.m. Significance was determined by two-way analysis of variance (ANOVA), with Bonferroni multiple-comparison analysis (B). ***P < 0.001.