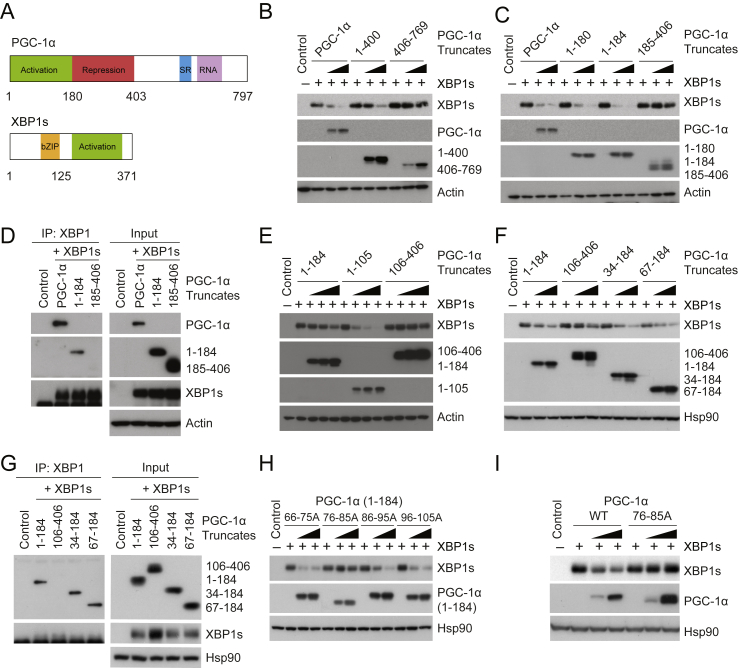
Figure 3.
Molecular interaction between XBP1s and the activation domain of PGC-1α. (A) The protein domains of mouse PGC-1α and XBP1s. (B) XBP1s, PGC-1α, PGC-1α (1-400), PGC-1α (406-769), and actin protein levels. Plasmid expressing XBP1s was transfected to HEK293 cells, along with increasing doses of plasmid producing full-length PGC-1α, PGC-1α (1-400) or (406-769). (C) Protein amounts of XBP1s, PGC-1α, PGC-1α (1-180), (1-184), (185-406), and actin. XBP1s plasmid was transfected into HEK293 cells, along with increasing amounts of plasmid expressing full-length PGC-1α, PGC-1α (1-180), (1-184), or (185-406). (D) Co-immunoprecipitation of XBP1s and different regions of PGC-1α. HEK293 cells were transfected with plasmids expressing XBP1s, together with flag-tagged full-length PGC-1α, PGC-1α (1-184), or (185-406). Co-immunoprecipitation was performed using anti-XBP1, and immunoprecipitates were analyzed with indicated antibodies and flag antibody (anti-flag) for PGC-1α (1-184) and (185-406) in immunoblot. (E) Levels of XBP1s, PGC-1α (1-184), (1-105), (106-406), and actin proteins in HEK293 cells transfected to express XBP1s plus increasing doses of indicated PGC-1α truncates. (F) XBP1s, PGC-1α (106-406), (34-184), (67-184), and Hsp90 protein levels. (G) Co-immunoprecipitation of XBP1s and different PGC-1α truncates. HEK293 cells expressing XBP1s, along with indicated flag-tagged PGC-1α constructs, were lysed and used for immunoprecipitation with anti-XBP1. (H) Immunoblot of XBP1s, of PGC-1α (1-184) constructs with indicated amino acids mutated to alanine, and of Hsp90 protein. HEK293 cells were transfected with XBP1s plasmid plus increasing doses of indicated PGC-1α (1-184) constructs. (I) Protein levels of XBP1s, wild-type (WT) and mutant PGC-1α (76-85A), and Hsp90. HEK293 cells were transfected with XBP1s plasmid plus increasing doses of plasmids encoding wild-type PGC-1α or mutated PGC-1α (76-85A). Each experiment was repeated at least two times.