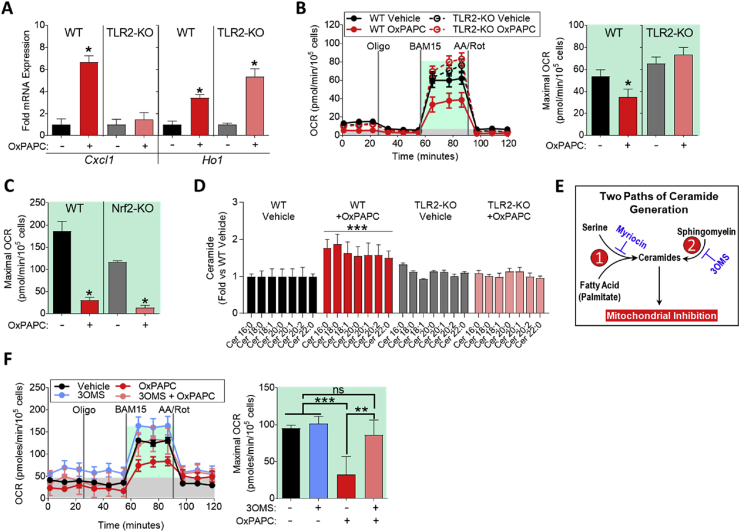
Figure 5.
Mitochondrial inhibition by OxPLs requires TLR2-dependent ceramide accumulation. A. mRNA expression of Cxcl1 and Ho1 measured by qPCR in WT or TLR2-KO BMDMs treated with vehicle or 10 μg/mL OxPAPC for 4 h (n = 3). B. MST of WT or TLR2-KO BMDMs treated with vehicle or 10 μg/mL OxPAPC for 4 h (n = 4). C. Maximal OCR calculated from the MST of WT (C57BL/6) or Nrf2-KO BMDMs treated with 10 μg/mL OxPAPC for 4 h (n = 4). D. Fold change in ceramide accumulation as measured by liquid chromatography–mass spectrometry (LC–MS) of WT or TLR2-KO BMDMs treated with vehicle or 30 μg/mL OxPAPC for 16 h (n = 5). Ceramides were quantified on an individual species basis, using the integrated peak area of the ion count. The integrated peak area of each analyte was normalized to the internal standard, Cer17:0, and to the protein content of each sample as determined by a Bradford assay. Significance was determined by two-way ANOVA of the ion count peak area. Data represented in as fold change compared to WT vehicle control. E. Summary of two pathways leading to ceramide generation in cells. Pathway 1, referred to as de novo ceramide biogenesis, contains the rate-limiting enzyme serine-palmitoyl-transferase 1, which is inhibited by myriocin. Pathway 2, referred to as sphingomyelin recycling, contains acidic and neutral sphingomyelinases as the rate-limiting enzymes. 3-O-methyl-sphingomyelin (3OMS) inhibits neutral sphingomyelinase. F. MST of WT BMDMs treated with 10 μg/mL OxPAPC and/or 1 μM 3-O-methyl-sphingomyelin (3OMS), an inhibitor of neutral sphingomyelinase, for 4 h (n = 4). Data are expressed as mean ± SEM. Biological replicates indicated by (n). Statistical significance calculated by Welch's 2-sided t-test (*p ≤ 0.05; **p < 0.01; ***p < 0.001).