Abstract
Ectopic adiposity has gained considerable attention because of its tight association with metabolic and cardiovascular health in persons with spinal cord injury (SCI). Ectopic adiposity is characterized by the storage of adipose tissue in non-subcutaneous sites. Magnetic resonance imaging (MRI) has proven to be an effective tool in quantifying ectopic adiposity and provides the opportunity to measure different adipose depots including intermuscular adipose tissue (IMAT) and intramuscular adipose tissue (IntraMAT) or intramuscular fat (IMF). It is highly important to distinguish and clearly define these compartments, because controversy still exists on how to accurately quantify these adipose depots. Investigators have relied on separating muscle from fat pixels based on their characteristic signal intensities. A common technique is plotting a threshold histogram that clearly separates between muscle and fat peaks. The cut-offs to separate between muscle and fat peaks are still not clearly defined and different cut-offs have been identified. This review will outline and compare the Midpoint and Otsu techniques, two methods used to determine the threshold between muscle and fat pixels on T1 weighted MRI. The process of water/fat segmentation using the Dixon method will also be outlined. We are hopeful that this review will trigger more research towards accurately quantifying ectopic adiposity due to its high relevance to cardiometabolic health after SCI.
Keywords: intermuscular adipose tissue, intramuscular adipose tissue, intramuscular fat, ectopic adiposity, magnetic resonance imaging
Introduction
Adipose tissue is a specialized loose connective tissue that is laden with adipocytes (Shen et al., 2003). These specialized cells express and secrete a multitude of hormones and proinflammatory cytokines (Trayhurn and Wood, 2004), which act in an autocrine, paracrine, and endocrine manner signaling the heart, musculoskeletal, central nervous and metabolic systems (Thalmann and Meier, 2007). Intermuscular adipose tissue (IMAT) is defined as the fat underneath the deep fascia and between adjacent muscle groups. Intramuscular adipose tissue (IntraMAT) is defined as the fat infiltrated between and/or within muscle fibers (Figure 1). Muscle and fat may contaminate within a voxel in severe fat infiltration cases such as patients with spinal cord injury (SCI) (Elder et al., 2004; Gorgey and Dudley, 2007). Therefore, IntraMAT may be less detectable and more difficult to accurately quantify in this population. IntraMAT is also referred to as interstitial adipose tissue (IAT; Mitsiopoulos et al., 1998). In the context of SCI, intramuscular fat (IMF) refers to the sum of both the fat within/between muscle fibers (IntraMAT) and between muscle groups (InterMAT) and should not be confused with IntraMAT. In this clinical population, the accumulation of IMF after injury has been linked to insulin sensitivity and glucose intolerance (Goodpaster et al., 2000; Elder et al., 2004). IMF and skeletal muscle atrophy of the thigh may account for up to 70% of glucose intolerance after SCI (Elder et al., 2004). Therefore, IMF (when accurately quantified) may serve as an important biomarker of metabolic disease in persons with SCI.
Figure 1.
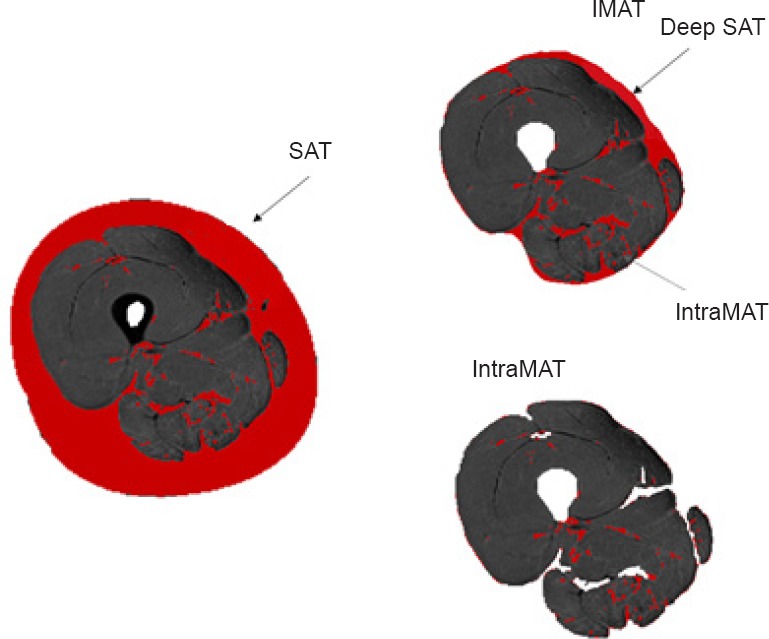
Auto-determined segmentation of subcutaneous adipose tissue (SAT), intermuscular adipose tissue (IMAT) and intramuscular adipose tissue (IntraMAT) of the thigh.
Mitsiopoulos et al. (1998) determined the validity of measuring IMAT and IntraMAT using both magnetic resonance imaging (MRI) and computerized tomography (CT). They compared the cross-sectional area (CSA) of skeletal muscle, interstitial and subcutaneous adipose tissue determined by MRI and CT to measurements in vivo. The report revealed a high correlation between the adipose tissue CSA of cadavers and the CSA acquired by MRI and CT (Mitsiopoulos et al., 1998). They concluded that both CT and MRI are acceptably precise measures of appendicular skeletal muscle, interstitial and subcutaneous adipose tissue (SAT). In comparison to CT, MRI has been shown to have a high sensitivity for identifying early adipose infiltration (Karampinos et al., 2012) and no associated risk of ionizing radiation (Smith-Bindman et al., 2009). Therefore, MRI is considered the “gold standard” technique for quantifying ectopic adipose tissue.
The purpose of this review is to investigate different techniques of quantifying IMAT/IntraMAT and IMF available in the current literature, as well as, to highlight the questionable points and inconsistencies within past studies involving adipose tissue quantification. We aim to compare the Otsu and Midpoint methods used to create bimodal histograms for pixel-to-pixel tissue segmentation using MRI.
Materials and Methods
Methodology for quantifying muscle, IMAT and IntraMAT in T1-weighted MRI
Before the study, the procedure, purposes, risks, and benefits associated with the study were explained and written consent was obtained. The experimental protocols were approved by the Human Research Ethics Committee of the Nagoya University Graduate School of Medicine and the Ethics Committee of the Research Center of Health, Physical Fitness, and Sports, Nagoya University. This study was completed in accordance with the Declaration of Helsinki.
The Otsu methods
Many previous studies have used histograms to separate muscle and adipose tissue based on pixel signal intensity (De Kerviler et al., 1996; Kent-Braun et al., 2000; Holmbäck et al., 2002; Elder et al., 2004; Gorgey and Dudley, 2007; Manini et al., 2007; Gorgey and Dudley, 2008). The software Medical Image Processing, Analysis and Visualization (MIPAV) isolates a region of interest (ROI) containing 50% muscle and 50% adipose tissue (Figure 2A; Manini et al., 2007). A bimodal histogram is created to determine the cut-off threshold between muscle and adipose tissue based on the signal intensity of each pixel. This process is repeated five times per MRI slice and the five threshold values are then averaged. The automated threshold is determined based on the peaks of the bimodal histogram of pixel intensities. Pixel intensity above the threshold value are classified as adipose tissue and below as muscle.
Figure 2.

Histogram versus Otsu methods.
(A) Medical Image Processing, Analysis and Visualization (MIPAV) isolates a region of interest (ROI) containing 50% of muscle and 50% adipose tissue. A midpoint threshold is calculated using pixel signal intensity. (B) The Otsu method of isolating six square ROIs. Three ROIs are selcted on the muscle tissue and three are selected from adipose tissue. An auto-determined threshold is calculated using MIPAV and pixel signal intensity. (C) Bimodal histogram using of Midpoint method showing the muscle and fat peaks. The apex of each peak is used as a point of determination before both peaks average to determine the magic point.
Akima et al. (2015, 2016) used the Otsu method to determine the threshold for separating muscle and fat pixels. First, 6 square ROIs are isolated on the magnetic resonance image (3 on muscle; 3 on adipose tissue; Figure 2B). A biomodal histogram is created based on the pixel intensities of the selected ROIs. Next, an auto-determined threshold is isolated, located at the base of the first peak of the bimodal histogram. This is repeated five times for each image slice and the average threshold value is used to classify tissue pixels; once more, pixel intensities above the threshold value are classified as adipose tissue and below as muscle. After determining the threshold value, individual muscles (e.g., the vastus group, rectus femoris, knee flexors, hips adductors, sartorius, and gracilis) and whole thigh muscle CSA are traced separating IMAT and IntraMAT depots. The CSA is determined by summing the respective tissue pixels and multiplying by the pixel surface area using various software programs. The pixel area is calculated based on the ratio of the field of view and the matrix size of the image.
The Midpoint method
The Midpoint method was previously used to quantify pixel signal intensity and distinguish between muscle and adipose tissue peaks (Gorgey and Dudley, 2007, 2008; Gorgey and Shepherd, 2010; Gorgey et al., 2012). The midpoint value is determined by selecting the five consecutive images of the mid-thigh (Lester et al., 2017). A circle is drawn around the entire thigh without touching the outer border; this is repeated for the next four images in the sequence. A bimodal histogram is created using pixel signal intensity. The trend of the histogram forms too distinct peaks along the x-axis (Figure 2C). The highest points of the two peaks (using the x values) are isolated and the average is taken. The average of these 5 averaged points is calculated; this is referred to as the midpoint average or “magic point”, or the point of separation between muscle and IMF for the entire thigh. After determining the midpoint average, the whole thigh muscle group, individual muscles, the subperiosteal CSA, both red and yellow marrows and SAT are traced along the anatomical borders separating IMF. CSA is then calculated by quantifying the number of tissue pixels within a specific region and multiplying by the pixel surface area. The Midpoint method is preferably used because it allows voxels of equal distribution of muscle and adipose tissue pixels to be split evenly among the muscle and fat peaks.
Methodology for quantifying IMAT and IntraMAT in water-fat images
The Dixon method is one of several MRI techniques for quantifying adipose tissue (Eggers and Börnert, 2014). Chemical shift-based water-fat separation is an alternative technique for evaluation of the fat fraction based on the different precession frequencies of water and lipid hydrogen protons (Dixon, 1984). The method of fat and water separation first described by Dixon exploits the difference in resonance precession frequencies of fat and water. Quantitative MRI techniques assessing the Intra-MAT have been validated and determined to provide an accurate, reliable calculation of IntraMAT, consistent with muscle biopsies (Smith et al., 2014). Furthermore, the best point of water and fat images can be calculated semi-automatically IMAT and IntraMAT ratio using both images. %Fat is calculated using the image intensity of both water image and fat image with the following formula:

However, chemical shift-based water-fat separation techniques require the implementation of advanced image processing algorithms that may not be available in all clinical settings (Alizai et al., 2012).
Comparison of Midpoint and Otsu methods
The Midpoint and Otsu methods are two commonly used techniques for quantifying adipose tissue using MRI. Magnetic resonance images from 10 abled-bodied individuals were randomly selected and analyzed using the two techniques. Threshold values and %IntraMAT of the knee extensor muscle group were determined using Midpoint and Otsu methods. Percent difference of IntraMAT between the two techniques was calculated. Independent t-tests were used to compare outputs and statistical significance was set to P < 0.05.
Results
Table 1 presents the threshold values and %IntraMAT of the knee extensor muscle group acquired from both the Midpoint and Otsu methods in 10 abled-bodied individuals. A statistically significant difference was observed between the outputs of both techniques P < 0.0001). %IntraMAT of the knee extensor muscle group varied greatly between the two techniques, in each of the 10 participants (Figure 3). Mean threshold value and %IntraMAT using the Midpoint method was 1,260 ± 77 and 0.25 ± 0.24%, respectively. Using the Otsu method, mean threshold value and %IntraMAT was 766.5 ± 63 and 6.05 ± 1.9%, respectively. The mean %difference between the two methods was 5.8 ± 1.8%.
Table 1.
A comparison between Midpoint and Otsu threshold techniques in quantifying intramuscular adipose tissue (IntraMAT) of the knee extensor muscle group in 10 abled-bodied subjects

Figure 3.
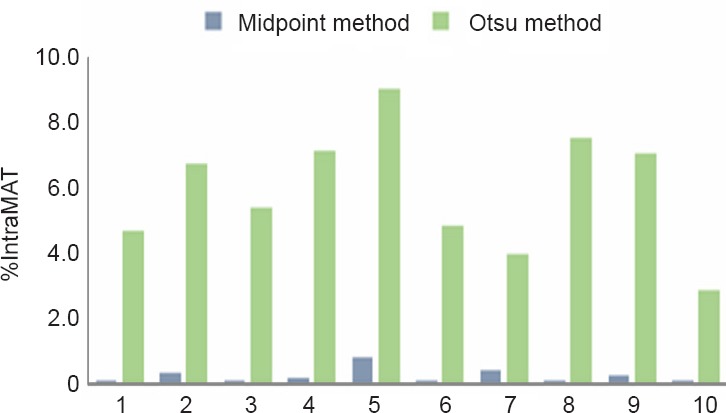
Comparison between the %IMF of the knee extensor muscle group calculated from the Midpoint and Otsu methods in 10 abled-bodied individuals (1–10).
The Midpoint method uses the midpoint between two peaks as the threshold separating muscle and fat. The Otsu method uses an auto-determined threshold based on the signal intensity at two peaks on a biomodal histogram.
Discussion
Classification of IMAT/IntraMAT and IMF
Addison et al. (2014) indicated the difficulty in investigating the properties of adipose tissues due to differences in classification method. For example, Mitsiopoulos et al. (1998) classified IntraMAT as interstitial adipose tissue (IAT) or the adipose tissue embedded in and between muscle fibers. Gorgey and Dudley (2007) classified the sum of IMAT/IntraMAT as IMF. They noted that IMF should not be confused with IMAT, which is the visible fat in and between muscle fibers. Because MRI and CT are not capable of separating between intra- and extramyocellular fat compartments (Kent-Braun et al., 2000; Ross et al., 2000; Ryan et al., 2002), the term IMF encompasses both adipose tissues. Elder et al. (2004) reported that to accurately quantify muscle atrophy, skeletal muscle CSA needs to be corrected for IMF. Failure to correct for IMF may result in underestimation of muscle atrophy by ~12% in SCI individuals (Elder et al., 2004). We have also noted during analysis of individual muscle CSA, IMF may encompass 20–30% of the total muscle size. For example, one can erroneously report that the knee extensor muscle CSA is 50 cm2 when in reality it is 35–40 cm2. Recently, Wade and Gorgey noted that the mid-thigh muscle CSA may be overestimated by 22% in persons with chronic SCI when failing to account for IMF (Wade and Gorgey, 2017).
Midpoint vs. Otsu Methods
The findings of the current report revealed a significant difference (P < 0.0001) between outputs of the Midpoint and Otsu methods. After analyzing 10 magnetic resonance images, the Otsu method generated a 60% higher mean threshold value than the Midpoint method. Because the cut-offs for muscle and fat were vastly different, this resulted in significantly different (P < 0.0001) values for knee extensor %IntraMAT. The current findings highlight the wide range of IntraMAT CSA values that may be generated when using various quantification methods; therefore, when analyzing data in longitudinal studies it is necessarily to remain consistent in the chosen methodology for adipose tissue quantification. The findings reveal a strike difference in outcomes acquired by the Midpoint and Otsu methods; however, we cannot conclude which of the two methods is more accurate at this point in time. Future studies should develop a methodological approach to truly determine the accuracy of each method in abled-bodied and SCI populations.
Adiposity and SCI
Extreme disuse from paralysis after SCI has shown to contribute to a concomitant loss of lean tissue and increased IMF in the lower extremity, because of reduced level of physical activity and autonomic nervous system dysfunction (Spungen et al., 2000; Gorgey and Dudley, 2007). IMF was observed to be 126% greater at 6 weeks post-SCI compared to abled-bodied controls. Over the following 3 months, IMF CSA continued to increase by 26% in SCI individuals. Excessive adipose tissue accumulation in non-subcutaneous sites imposes significant health risks to the SCI population, leading to poor quality of life (Gater, 2007). The storage of triglycerides in intramuscular sites is linked to chronic inflammation, increased total cholesterol, and decreased strength and mobility (Kershaw and Flier, 2004; Addison et al., 2014). Adipose tissue is considered by many as the largest endocrine gland in the body; endocrine properties include the secretion of regulatory proteins such as leptin, cytokines and adiponectin which are likely to influence whole body metabolism, energy intake as well as insulin sensitivity (Kershaw and Flier, 2004; Addison et al., 2014). Elder et al. (2004) reported that IMF and skeletal muscle atrophy in the thigh accounted for 70% of glucose intolerance after SCI. The inflammatory nature of adipose tissue has been linked to comorbidities including obesity, type II diabetes mellitus, cancer and cardiovascular disease. The prevalence of obesity within the SCI population may exceed two-thirds and as much as 50% of this population may suffer from excessive adiposity that exceeds 30% of total body mass, despite having normal body mass index (Spungen et al., 2003; Gorgey and Gater, 2011). In terms of rehabilitation, IMF has been shown to mechanically impede the progression of current within muscles during surface neuromuscular electrical stimulation (Gorgey et al., 2013a). This may result in an excess of current amplitude and lead to rapid muscle fatigue of the paralyzed muscles.
In addition to IMAT/IntraMAT, ectopic adipose tissue may infiltrate the bone marrow of long bones. Initial observations by Gorgey et al. (2013b) showed that persons with motor complete SCI had 2–3 times more bone marrow adipose tissue compared to matched able-bodied controls, primarily because of unloading of the lower extremities. Moreover, bone marrow adipose tissue was inversely related to bone mineral density and bone mineral content as measured by dual-energy X-ray absorptiometry (DXA). This link may contribute to a greater understanding of pathways that lead to the development of osteoporosis after SCI (Gorgey et al., 2013b). Gorgey et al. (2013b) has shown that bone marrow adipose tissue can be effectively quantified using the Midpoint method; however, this ROI has yet to be quantified using the Otsu technique.
Limitations of IMAT/IntraMAT measurement by MRI
With respect to measurement of IMAT/IntraMAT in T1 weighted images, the magnetic field is not constant; therefore, it may be necessary to correct images before analysis (Elder et al., 2004; Manini et al., 2007; Popadic Gacesa et al., 2011, 2014; Buford et al., 2012; Akima et al., 2015). Previous studies used a well-established intensity normalization algorithm to determine that image heterogeneity can be linked to suboptimal radiofrequency coil uniformity or gradient-driven eddy currents (Sled et al., 1998; Manini et al., 2007; Akima et al., 2015). A poor signal-to-noise ratio often results in images with skewed contrast (Smith et al., 2004), resulting in inaccurate pixel signal intensities. If the signal intensity of an image is skewed from the true value, muscle and/or fat can be over- or underestimated. Additionally, persons with SCI are known to have frequent muscle spasms which may take place during scanning, resulting in additional noise. Spasticity may lead to an imaging artifact which results in inaccurate quantification of muscle and IMF CSAs. Moreover, muscles of the thigh are often severely atrophied making it more difficult to precisely define anatomical borders of different muscle groups. A person's levels of injury, as well as the time since injury are two factors amongst many that can affect muscle CSA and image quality (Gorgey and Dudley, 2007). Muscles with significant deterioration and infiltration of fat require a strong understanding of the anatomy to precisely trace muscle groups and fat depots. Therefore, it may be benefical to quantify IMAT/IntraMAT using fully-automated threshold techniques (Orgiu et al., 2016). Providing automated software is likely to account for subjectivity and error that may occur with manual tracing technique.
Conclusion
Investigators differ in their classification and analysis methods of IMAT/IntraMAT. The cut-offs to separate between muscle and fat peaks are still not clearly defined and different cut-offs have been identified. There are a variety of available MRI analyzing techniques, many of which have been validated in the literature. T1-weighted and water-fat images have both been used for measurement of muscle and adipose tissue. Different adipose tissue compartments have been identified in the current review; however, their full contribution to overall metabolic health is not fully understood. Future research should clearly define the cut-offs between muscle and adipose tissue to accurately quantify different depots of ectopic adiposity.
Acknowledgments
We thank Hunter Holmes McGuire Research Institute and Spinal Cord Injury Services and Disorders for providing the environment to conduct clinical human research trials, as well as, all the MRI technicians who assisted in the process of performing scans. ASG is currently supported by the Department of Veteran Affairs, Veteran Health Administration, Rehabilitation Research and Development Service (B7867-W) and DoD-CDRMP (W81XWH-14-SCIRP-CTA).
Footnotes
Conflicts of interest: None declared.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer review report:
Reviewer: Viness Pillay, University of the Witwatersrand, France.
Comments to authors: In this review, the authors have looked at approaches towards more accurately quantifying ectopic adiposity, highlighting inconsistences of past approaches. The authors also compared the Otsu and Midpoint methods to create bimodal histograms for pixel-to-pixel tissue segmentation using MRI. This review is of high interest and significance because of the relation of adiposity with metabolic and cardiovascular health.
Funding: This study was supported in part by Grant-in-Aid for JSPS Research Fellow.
References
- Addison O, Marcus RL, Lastayo PC, Ryan AS. Intermuscular fat: a review of the consequences and causes. Int J Endocrinol. 2014;2014:309570. doi: 10.1155/2014/309570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akima H, Hioki M, Yoshiko A, Koike T, Sakakibara H, Takahashi H, Oshida Y. Intramuscular adipose tissue determined by T1-weighted MRI at 3T primarily reflects extramyocellular lipids. Magn Reson Imaging. 2016;34:397–403. doi: 10.1016/j.mri.2015.12.038. [DOI] [PubMed] [Google Scholar]
- Akima H, Yoshiko A, Hioki M, Kanehira N, Shimaoka K, Koike T, Sakakibara H, Oshida Y. Skeletal muscle size is a major predictor of intramuscular fat content regardless of age. Eur J Appl Physiol. 2015;115:1627–1635. doi: 10.1007/s00421-015-3148-2. [DOI] [PubMed] [Google Scholar]
- Alizai H, Nardo L, Karampinos DC, Joseph GB, Yap SP, Baum T, Krug R, Majumdar S, Link TM. Comparison of clinical semi-quantitative assessment of muscle fat infiltration with quantitative assessment using chemical shift-based water/fat separation in MR studies of the calf of post-menopausal women. Eur Radiol. 2012;22:1592–1600. doi: 10.1007/s00330-012-2404-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buford TW, Lott DJ, Marzetti E, Wohlgemuth SE, Vandenborne K, Pahor M, Leeuwenburgh C, Manini TM. Age-related differences in lower extremity tissue compartments and associations with physical function in older adults. Exp Gerontol. 2012;47:38–44. doi: 10.1016/j.exger.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kerviler E, Leroy-Willig A, Duboc D, Eymard B, Syrota A. MR quantification of muscle fatty replacement in McArdle's disease. Magn Reson Imaging. 1996;14:1137–1141. doi: 10.1016/s0730-725x(96)00236-6. [DOI] [PubMed] [Google Scholar]
- Dixon WT. Simple proton spectroscopic imaging. Radiology. 1984;153:189–194. doi: 10.1148/radiology.153.1.6089263. [DOI] [PubMed] [Google Scholar]
- Eggers H, Börnert P. Chemical shift encoding-based water-fat separation methods. J Magn Reson Imaging. 2014;40:251–268. doi: 10.1002/jmri.24568. [DOI] [PubMed] [Google Scholar]
- Elder CP, Apple DF, Bickel CS, Meyer RA, Dudley GA. Intramuscular fat and glucose tolerance after spinal cord injury--a cross-sectional study. Spinal Cord. 2004;42:711–716. doi: 10.1038/sj.sc.3101652. [DOI] [PubMed] [Google Scholar]
- Gater DR., Jr Obesity after spinal cord injury. Phys Med Rehabil Clin N Am. 2007;18:333–351. doi: 10.1016/j.pmr.2007.03.004. vii. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71:885–892. doi: 10.1093/ajcn/71.4.885. [DOI] [PubMed] [Google Scholar]
- Gorgey AS, Dudley GA. Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord. 2007;45:304–309. doi: 10.1038/sj.sc.3101968. [DOI] [PubMed] [Google Scholar]
- Gorgey AS, Dudley GA. Spasticity may defend skeletal muscle size and composition after incomplete spinal cord injury. Spinal Cord. 2008;46:96–102. doi: 10.1038/sj.sc.3102087. [DOI] [PubMed] [Google Scholar]
- Gorgey AS, Shepherd C. Skeletal muscle hypertrophy and decreased intramuscular fat after unilateral resistance training in spinal cord injury: case report. J Spinal Cord Med. 2010;33:90–95. doi: 10.1080/10790268.2010.11689681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgey AS, Gater DR. Regional and relative adiposity patterns in relation to carbohydrate and lipid metabolism in men with spinal cord injury. Appl Physiol Nutr Metab. 2011;36:107–114. doi: 10.1139/H10-091. [DOI] [PubMed] [Google Scholar]
- Gorgey AS, Mather KJ, Cupp HR, Gater DR. Effects of resistance training on adiposity and metabolism after spinal cord injury. Med Sci Sports Exerc. 2012;44:165–174. doi: 10.1249/MSS.0b013e31822672aa. [DOI] [PubMed] [Google Scholar]
- Gorgey AS, Cho GM, Dolbow DR, Gater DR. Differences in current amplitude evoking leg extension in individuals with spinal cord injury. NeuroRehabilitation. 2013a;33:161–170. doi: 10.3233/NRE-130941. [DOI] [PubMed] [Google Scholar]
- Gorgey AS, Poarch HJ, Adler RA, Khalil RE, Gater DR. Femoral bone marrow adiposity and cortical bone cross-sectional areas in men with motor complete spinal cord injury. PM R. 2013b;5:939–948. doi: 10.1016/j.pmrj.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Holmbäck AM, Askaner K, Holtås S, Downham D, Lexell J. Assessment of contractile and noncontractile components in human skeletal muscle by magnetic resonance imaging. Muscle Nerve. 2002;25:251–258. doi: 10.1002/mus.10031. [DOI] [PubMed] [Google Scholar]
- Karampinos DC, Baum T, Nardo L, Alizai H, Yu H, Carballido-Gamio J, Yap SP, Shimakawa A, Link TM, Majumdar S. Characterization of the regional distribution of skeletal muscle adipose tissue in type 2 diabetes using chemical shift-based water/fat separation. J Magn Reson Imaging. 2012;35:899–907. doi: 10.1002/jmri.23512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent-Braun JA, Ng AV, Young K. Skeletal muscle contractile and noncontractile components in young and older women and men. J Appl Physiol (1985) 2000;88:662–668. doi: 10.1152/jappl.2000.88.2.662. [DOI] [PubMed] [Google Scholar]
- Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- Lester RM, Johnson K, Khalil RE, Khan R, Gorgey AS. MRI analysis and clinical significance of lower extremity muscle cross-sectional area after spinal cord injury. Neural Regen Res. 2017;12:714–722. doi: 10.4103/1673-5374.206634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manini TM, Clark BC, Nalls MA, Goodpaster BH, Ploutz-Snyder LL, Harris TB. Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am J Clin Nutr. 2007;85:377–384. doi: 10.1093/ajcn/85.2.377. [DOI] [PubMed] [Google Scholar]
- Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985) 1998;85:115–122. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- Orgiu S, Lafortuna CL, Rastelli F, Cadioli M, Falini A, Rizzo G. Automatic muscle and fat segmentation in the thigh from T1-Weighted MRI. J Magn Reson Imaging. 2016;43:601–610. doi: 10.1002/jmri.25031. [DOI] [PubMed] [Google Scholar]
- Popadic Gacesa JZ, Kozic DB, Grujic NG. Triceps brachii strength and regional body composition changes after detraining quantified by MRI. J Magn Reson Imaging. 2011;33:1114–1120. doi: 10.1002/jmri.22548. [DOI] [PubMed] [Google Scholar]
- Popadic Gacesa JZ, Secher NH, Momcilovic M, Grujic NG. Association between intramuscular fat in the arm following arm training and INSIG2. Scand J Med Sci Sports. 2014;24:907–912. doi: 10.1111/sms.12102. [DOI] [PubMed] [Google Scholar]
- Ross R, Goodpaster B, Kelley D, Boada F. Magnetic resonance imaging in human body composition research. From quantitative to qualitative tissue measurement. Ann N Y Acad Sci. 2000;904:12. doi: 10.1111/j.1749-6632.2000.tb06415.x. 17. [DOI] [PubMed] [Google Scholar]
- Ryan AS, Dobrovolny CL, Smith GV, Silver KH, Macko RF. Hemiparetic muscle atrophy and increased intramuscular fat in stroke patients. Arch Phys Med Rehabil. 2002;83:1703–1707. doi: 10.1053/apmr.2002.36399. [DOI] [PubMed] [Google Scholar]
- Shen W, Wang Z, Punyanita M, Lei J, Sinav A, Kral JG, Imielinska C, Ross R, Heymsfield SB. Adipose tissue quantification by imaging methods: a proposed classification. Obes Res. 2003;11:5–16. doi: 10.1038/oby.2003.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Smith-Bindman R, Lipson J, Marcus R, Kim KP, Mahesh M, Gould R, Berrington de González A, Miglioretti DL. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169:2078–2086. doi: 10.1001/archinternmed.2009.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC, Parrish TB, Abbott R, Hoggarth MA, Mendoza K, Chen YF, Elliott JM. Muscle-fat MRI: 1.5 Tesla and 3.0 Tesla versus histology. Muscle Nerve. 2014;50:170. doi: 10.1002/mus.24255. 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Spungen AM, Wang J, Pierson RN, Jr, Bauman WA. Soft tissue body composition differences in monozygotic twins discordant for spinal cord injury. J Appl Physiol 1985. 2000;88:1310–1315. doi: 10.1152/jappl.2000.88.4.1310. [DOI] [PubMed] [Google Scholar]
- Spungen AM, Adkins RH, Stewart CA, Wang J, Pierson RN, Jr, Waters RL, Bauman WA. Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol (1985) 2003;95:2398–2407. doi: 10.1152/japplphysiol.00729.2002. [DOI] [PubMed] [Google Scholar]
- Thalmann S, Meier CA. Local adipose tissue depots as cardiovascular risk factors. Cardiovasc Res. 2007;75:690–701. doi: 10.1016/j.cardiores.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- Wade RC, Gorgey AS. Anthropometric prediction of skeletal muscle cross-sectional area in persons with spinal cord injury. J Appl Physiol (1985) 2017;122:1255–1261. doi: 10.1152/japplphysiol.01042.2016. [DOI] [PubMed] [Google Scholar]


