Abstract
Dental caries is a disease that results from microbiome dysbiosis with the involvement of multiple cariogenic species, including mutans streptococci (MS), lactobacilli, Scardovia wiggsiae, and several Actinomyces species that have the cariogenic traits of acid production and acid tolerance. Sugar consumption also plays an important role interacting with microbiome dysbiosis, determining the fate of caries development. In addition, the MS transmission that encompasses multiple sources can have long-term impacts on the oral microbiome and caries development in children. Intervention in MS transmission in early childhood may promote effective long-term caries prevention. Anticaries regimens aimed against the above mechanisms will be important for successful caries management. Xylitol and erythritol may serve as good components of anticaries regimens as oral microbiome modifiers, sugar substitutes, and agents to prevent MS transmission in early childhood with both oral and systemic benefits. Further studies are needed to elucidate the mechanism of the anticaries effects of xylitol and erythritol with consideration of their impacts on the microbiome and bacterial virulence, in addition to cariogenic bacteria levels as well as their benefits for overall health. On the other hand, the anticaries agent C16G2, specifically targeting Streptococcus mutans, the most common cariogenic bacterial species, has shown good safety for short-term oral topical use and promising effects in reducing S. mutans in vitro and in vivo with the promotion of oral commensal bacteria. Future study on its anticaries effect will need to include its long-term impact on the oral microbiome and effects on other important cariogenic bacteria.
Keywords: microbial ecology, caries detection/diagnosis/prevention, caries treatment, bacterial virulence, infectious disease(s), antibacterial agents
Introduction
Dental caries is a multifactorial infectious disease that is characterized by microbiome dysbiosis with the elevation of cariogenic bacteria (Loesche 1986; Tanner et al. 2016). In the oral cavity, tooth surfaces are always covered by dental plaque. In the presence of sucrose and other fermentable carbohydrates, dynamic mineral loss (demineralization) is initiated by acid produced by oral bacteria metabolizing fermentable carbohydrates. The demineralized tooth structure can be repaired by remineralization when acid production diminishes as carbohydrate substrates are exhausted or acids are neutralized by intrinsic or extrinsic buffering agents in dental plaque. Balancing the equilibrium between demineralization and remineralization is the key for initiation, progression, or reversal of dental caries (Takahashi and Nyvad 2011; Young and Featherstone 2013).
Although multiple biological risk factors contribute to the caries balance, microbiome dysbiosis interacting with diet plays a critical role in the fate of dental caries development (Takahashi and Nyvad 2011; Tanner et al. 2016; Young and Featherstone 2013). Restorative dental treatment does not alter the cariogenic bacteria loading (microbiome dysbiosis) in the rest of the mouth and is often followed by continuing caries development in high-caries-risk populations (Zhan et al. 2006; Tanner, Kent, et al. 2011; Featherstone et al. 2012; Hughes et al. 2012; Chaffee et al. 2016). Therefore, anticaries treatments aimed to rebalance the dysbiosis of the oral microbiome are needed for successful caries management and prevention. This article focuses on microbiome dysbiosis related to dental caries, discusses factors influencing the microbiome dysbiosis that lead to caries development, and comments on anticaries approaches by either targeted antimicrobial treatment against Streptococcus mutans, one of the main cariogenic bacteria, or polyols, such as xylitol and erythritol that are aimed at correcting oral microbiome dysbiosis.
Microbiome Dysbiosis in Dental Caries
The mutans streptococci (MS) group is the most studied and well-accepted cariogenic bacteria collection of species. Both culture-based and DNA/RNA-based studies have confirmed MS as one of the main cariogenic bacterial groups, with S. mutans and Streptococcus sobrinus as the 2 main subspecies in humans (Loesche 1986; Tanzer et al. 2001; Tanner, Kent, et al. 2011; Tanner, Mathney, et al. 2011; Hughes et al. 2012; Tanner 2015; Tanner et al. 2016):
elevated oral MS levels have been detected in subjects prior to dental caries development (Leverett et al. 1993),
high MS levels have been observed in dental plaque or stimulated saliva in children or adults with initial or active decay (Loesche 1986; Tanner, Mathney, et al. 2011; Tanner et al. 2012),
clinical trials have shown that reduction or elimination of MS resulted in caries prevention and relapse of MS dysbiosis resulted in new caries development (Tanzer et al. 2001; Zhan et al. 2006; Featherstone et al. 2012; Laitala et al. 2013), and
animal studies indicated that MS inoculation induced the most amount of dental caries in both smooth surfaces and pits and fissures (Loesche 1986).
Therefore, there has been considerable attention given to seeking treatment regimens to reduce or eliminate MS for caries prevention.
However, recent DNA/RNA-based studies have also expanded the microbiology in dental caries to include not only more cariogenic bacterial species but also the commensal bacteria associated with healthy subjects. Tanner et al. recently presented a comprehensive review on the caries microbiome summarizing projects conducted in the Forsyth Institute in Boston, United States. Their studies indicated that dental caries is a result of dysbiosis of acid-producing and acid-tolerant bacteria with a close relationship to a frequent sugary or carbohydrate diet. In addition to the strong correlation of MS with dental caries (Hughes et al. 2012; Tanner et al. 2012), they also indicated a wide diversity of the caries microbiome, including the association of Actinomyces and related species with caries (Tanner 2015), especially a new species, Scardovia wiggsiae, in the Actinomyces/Bifidobacterium family and several other Actinomyces species (Hughes et al. 2012; Kressirer et al. 2017). Hence, anticaries treatment may be more logical in focusing on correcting the microbiome dysbiosis than on eliminating a single pathological species. Further studies are also needed to confirm the roles of other cariogenic bacteria in caries initiation, progression, and management as well as to identify effective chair-side tools to monitor caries microbiome dysbiosis and to guide clinicians to effectively manage dental caries chemically.
Targeted Antimicrobial Treatment against Streptococcus mutans
A pheromone-guided “smart” antimicrobial peptide—namely, a specifically targeted antimicrobial peptide C16G2—has been developed for caries prevention. C16G2 is targeted at killing S. mutans, the most prevalent MS species in humans, based on the concept that this will lead to rebalancing the caries microbiome dysbiosis for an effective anticaries effect. C16G2 consists of a targeting domain that is a truncated S. mutans competence stimulating peptide, which bonds effectively on S. mutans cells, and a killing domain, G2, a truncated broad-spectrum killing peptide (novispirin G10). The 2 domains are linked by a flexible triglycine linker region (Eckert et al. 2012). C16G2 targetedly kills S. mutans without a significant effect on other commensal bacteria in vitro (Eckert et al. 2012; Guo et al. 2015). Use of C16G2 rinse for 4 d significantly reduced S. mutans in plaque and saliva, accompanied by an increase of Streptococcus sanguinis and Streptococcus gordonii levels, resting pH, and reduced acid formation in dental plaque in vivo (Eckert et al. 2012). The completed phase 1 and 2 studies showed good safety data for C16G2 in the oral cavity and confirmed that S. mutans levels were rapidly reduced and remained low, even after cessation of therapy (Eckert et al., unpublished data). These results show the potential of the effectiveness of targeted treatment on caries prevention and its benefits on rebalancing the oral microbiome through targeting the main cariogenic bacteria.
One concern is that C16G2 only targets S. mutans while caries microbiome dysbiosis involves multiple cariogenic species. For example, S. sobrinus, the other main species in the MS group, is commonly found in high-risk individuals (Loesche 1986; Köhler et al. 1995; Tanzer et al. 2001). Studies suggest that S. sobrinus may be more cariogenic than S. mutans. S. sobrinus strains are usually more acidogenic, produce structurally different and more abundant polysaccharide, and adhere better to smooth tooth surfaces (Köhler et al. 1995). The presence of S. sobrinus might be a stronger indicator of future caries risk and might place the patients at higher risk than S. mutans alone. In addition, newly detected S. wiggsiae and several Actinomyces species have been shown to play important roles in caries formation when MS is absent (Hughes et al. 2012; Kressirer et al. 2017). In these cases, a targeted treatment only against S. mutans may not be effective. Second, in vitro and pilot studies have shown effective elimination of S. mutans from the oral biofilm by C16G2. It may also be important to consider that S. mutans is a part of the normal indigenous oral flora. Therefore, further monitoring on potential microbiome ecology consequences after elimination of S. mutans from oral flora is needed. The focus of anticaries treatment may be best achieved by rebalancing the dysbiosis rather than elimination of S. mutans or any other single species. These concerns will need to be investigated in the phase 3 clinical studies in humans.
Sugar Intake and the Microbiome Dysbiosis
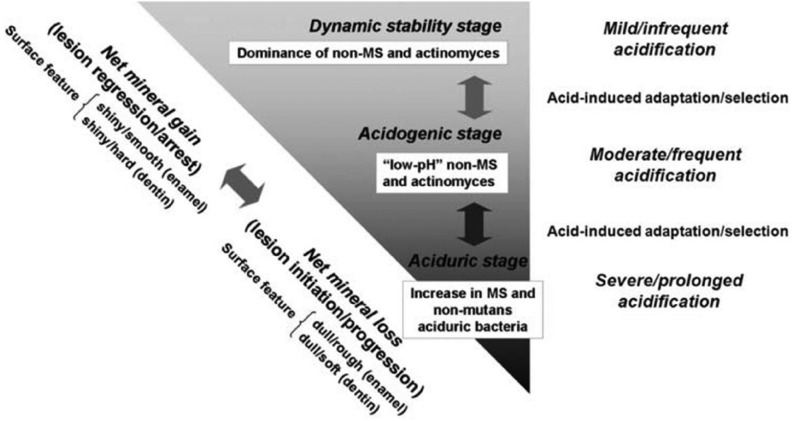
Frequent carbohydrate ingestion plays a significant role in modifying the oral microbiome. Takahashi and Nyvad (2011) proposed an ecological hypothesis for caries microbiome dysbiosis with 3 reversible stages (Fig. 1):
Figure 1.
The caries process according to an extended caries ecological hypothesis (Takahashi and Nyvad 2011). MS, mutans streptococci.
a dynamic stable stage on sound enamel where plaque contains mainly nonmutans streptococci and Actinomyces with mild and infrequent acidification and demineralization/remineralization shifting toward net mineral gain;
an acidogenic stage when increasing sugar/fermentable carbohydrate frequency results in aciduric and acidogenic adaptation of the nonmutans bacteria, a selective shift of aciduric bacteria, and a shift of demineralization/remineralization balance toward net mineral loss and initiation/progression of dental caries;
an aciduric stage, in which prolonged acidic conditions result in predominant colonization of aciduric and acidogenic bacteria, including mutans streptococci, lactobacilli, aciduric nonmutans streptococci, Actinomyces, bifidobacteria, and yeasts, as well as prolonged net mineral loss and progression of dental caries.
This hypothesis is supported by the observations that, on sound enamel surfaces or in caries-free subjects, there are generally low levels of MS, lactobacilli, and other aciduric nonmutans streptococci in dental plaque with a less acidogenic and aciduric environment (Loesche 1986; Tanzer et al. 2001; Khoo et al. 2005). The aciduric and acidogenic bacteria, including MS and nonmutans species, increase in early enamel white spot lesions or before clinical lesions are detected (Leverett et al. 1993; Takahashi and Nyvad 2011; Tanner et al. 2012; Simón-Soro et al. 2013). Last, in subjects with active caries, MS and nonmutans aciduric and acigodgenic bacteria become dominant, accompanied by decreased microbiome diversity (Loesche 1986; Tanner, Mathney, et al. 2011; Tanner et al. 2012). Frequent fermentable carbohydrate and sucrose intake plays a leading role in the shift of caries microbiome dysbiosis to more aciduric flora with an increase of MS and lactobacilli and a decrease of S. sanguinis while restricted sugar in the diet lowered the salivary lactobacilli and plaque MS (Loesche 1986; Tanzer et al. 2001; Takahashi and Nyvad 2011; Simón-Soro et al. 2013).
In addition to acid formation, sucrose also influences the microbiome by providing the main substrate for glucosyltransferases (GTFs) to synthesize extracellular polysaccharide (EPS). EPS formed in situ provides a matrix to enmesh the microorganisms in 3-dimensional multicellular structures that are firmly attached to teeth. The EPS-enriched biofilm limits diffusion, prolongs acidification processes in the presence of sugar, and creates a barrier to protect bacteria from antimicrobial treatment (Loesche 1986; Simón-Soro et al. 2013). Given the significant roles of sugar in caries formation and caries microbiome dysbiosis, sugar substitutes should be a significant part of anticaries therapy in high-risk subjects.
Transmission of MS and Its Relationship to Microbiome Dysbiosis
Timing for transmission and colonization of MS also affects microbiome dysbiosis. For high-risk young children, the critical time for MS transmission and colonization is from birth to 3 y (Li and Caufield 1995; Wan et al. 2003). Early colonization of S. sanguinis was associated with delayed MS colonization, and MS colonization can be accompanied by decreased levels of commensal bacteria such as S. sanguinis (Caufield et al. 2000; Simón-Soro et al. 2013). Furthermore, children with early colonization of MS at or before age 4 y not only had high levels of MS but were also more likely to develop new caries than those who did not colonize or colonized later (Alaluusua and Renkonen 1983). Therefore, early intervention to delay MS transmission can be a strategy for long-term caries prevention in children. Studies on maternal use of xylitol have shown strong evidence for delaying and reducing MS colonization as well as long-term caries prevention in children (Söderling 2009).
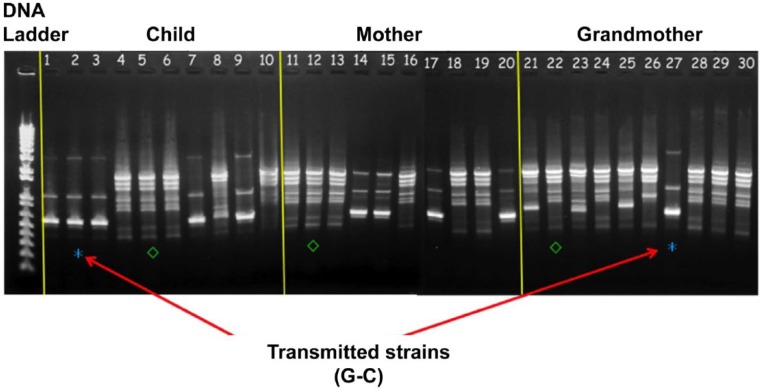
However, although transmission of MS from mother to child is widely accepted as a main source of early MS acquisition in children (Li and Caufield 1995), the maternal transmission rate of MS ranges from 33% to 100%, with other sources of MS transmission being documented within and beyond the family (Li and Caufield 1995; Lindquist and Emilson 2004; Mitchell et al. 2009; Doméjean et al. 2010; Zhan, Tan, et al. 2012). MS transmission from father to child and between spouses is well documented (Emanuelsson 2001). Our group studied multigenerational MS transmission in young children with early childhood caries in maternal-grandmother-mother-child triads. Ten maternal-grandmother-mother-child triads were recruited with maternal grandmothers as the primary caregivers for the children. Saliva samples were collected from each member of the triads, and MS were cultured on Mitis Salivarius Sucrose Bacitracin agar. Five MS colonies were isolated for arbitrarily primed (AP)–polymerase chain reaction (PCR) assay with primers OPA-5 and OPA-13 for genotyping. The results demonstrated all possible MS transmission combinations (Fig. 2) in the triads with the grandmother-child transmission rate as the highest as 55% (Table). Other studies have also demonstrated horizontal MS transmission in nursery schools and elementary school from playmates who had close contact with the child (Doméjean et al. 2010). Recently, 2 studies demonstrated that ~90% of MS genotypes in 2- to 5-y-old children with early childhood caries were from nonmaternal sources even when mothers were the primary caregivers (Mitchell et al. 2009; Zhan, Tan, et al. 2012). These results highlight the importance of nonmaternal bacterial transmission routes and the complexity of MS transmission in young children. MS transmission follows the general rule of transmission in infectious disease—specifically, that when close contact is present, MS from any source can infect children. In the early life of infancy, MS transmission may mainly occur from family members and caregivers such as mothers, fathers, grandparents, and siblings. As the social contact of the child extends beyond the family, horizontal transmission from peer playmates becomes more prominent. Children who are placed in environments such as nurseries, daycare centers, or elementary schools for extended periods of time favor horizontal transmission from child to child. Therefore, prevention of MS transmission should be considered in a more broadened perspective and may be an effective path in the prevention of caries microbiome dysbiosis in children and adults at high risk for MS transmission. A summary of transmission routes is illustrated in Figure 3.
Figure 2.
Arbitrarily primed–polymerase chain reaction (PCR) results: an example of 1 triad with mutans streptococci (MS) transmission, using OPA-5 primer. Lanes 1 to 10 show isolates from the child, lanes 11 to 20 are isolates from the mother, and lanes 21 to 30 are isolates from the grandmother. (*) indicates a shared genotype between the grandmother and the child (transmission). (◊) indicates another uniquely shared genotype between the grandmother, the mother, and the child.
Table.
Distribution of Sources of Mutans Streptococci Transmission among Grandmother-Mother-Child Triads.
| Transmission Pattern | No./Total Groups | % |
|---|---|---|
| G-M-C | 1/9 | 11 |
| G-M | 1/9 | 11 |
| M-C | 3/10 | 30 |
| G-C | 4/9 | 44 |
C, child; G, grandmother; M, mother. Only 9 grandmothers had mutans streptococci (MS) infection while all 10 mothers or children in the triads had MS infection.
Figure 3.
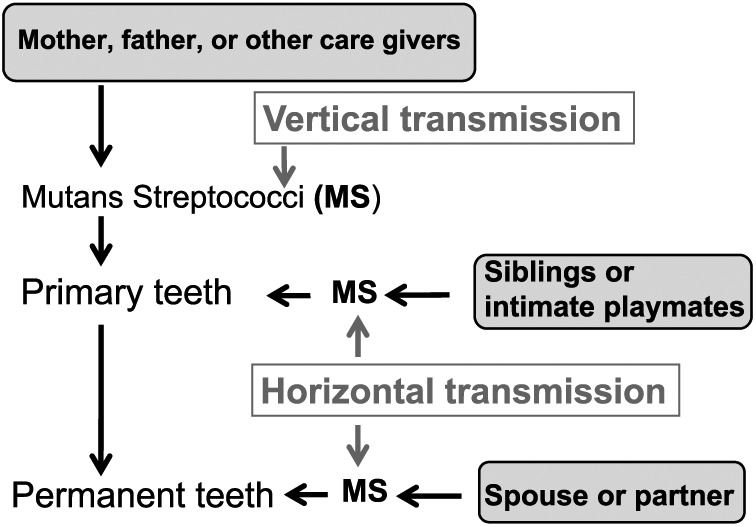
Routes of mutans streptococci (MS) transmission (Takahashi and Nyvad 2011). Vertical transmission of MS refers to MS transmitted from the mother, the father, or other caregivers to a child. Horizontal transmission refers to MS transmitted from siblings, intimate playmates, or close partners or spouses to a child or an adult.
Erythritol and Xylitol in Caries Prevention
Since sugar intake plays a significant role in caries induction and dysbiosis of the microbiome, sugar substitutes, especially polyols, can play significant roles in combating dental caries by reducing acid production, EPS formation, and plaque accumulation and therefore modify caries microbiome dysbiosis.
Current approaches of using polyols for caries prevention focus on topical uses such as chewing gum, lozenges, and wipes with evidence of the anticariogenic effects of chewing sorbitol, xylitol, or sorbitol/xylitol gum (Mickenautsch et al. 2007). Xylitol is the most studied polyol with moderate evidence on the anticaries effect of topical xylitol use over 4 g/d compared to other polyols or fluoride varnish (Janakiram et al. 2017). Recent studies also demonstrated a better anticaries effect of erythritol, a nonnutritive 4-carbon polyol with similar sweetness to sucrose, less laxative side effects compared to other polyols, and potential cardiovascular benefits, especially to diabetic patients, than xylitol/sorbitol (de Cock et al. 2016).
The mechanisms of xylitol/erythritol anticaries activity include decreased dental plaque formation and reduced adherence of common oral streptococci, inhibited growth of cariogenic bacteria and GTF activities, and decreased expression of bacterial genes involved in sucrose metabolism (Milgrom et al. 2012; Zhan, Featherstone, et al. 2012; de Cock et al. 2016). Short-term (6 mo or less) daily xylitol dose over 6 g has been reported to reduce oral MS levels (Holgerson et al. 2007), while long-term (over 12 mo) xylitol use on MS showed mixed results (Zhan, Cheng, et al. 2012). Habitual use of xylitol can induce xylitol-resistant MS. It was hypothesized that long-term xylitol use may select for xylitol-resistant MS with less virulence, leading to unchanged MS levels in some studies (Söderling 2009). However, the effects of xylitol on MS virulence using xylitol-resistant or xylitol-sensitive MS have shown conflicting results on their cariogenicity (Zhan, Featherstone, et al. 2012).
An important anticaries effect of xylitol is that maternal use of xylitol prevents MS transmission/colonization in early childhood with a long-term impact on the oral microbiome and anticaries effects in children (Söderling 2009). As MS transmission has multiple sources, prevention of MS transmission may be more effective if it is child centered. We explored the effect of direct use of xylitol wipes in high-risk infants on their MS colonization and found it significantly reduced caries formation in the infants but failed to prevent MS colonization in this population (Zhan, Cheng, et al. 2012; Zhan, Featherstone, et al. 2012). In the light that xylitol also did not reduce the MS levels in mothers but reduced the MS transmission to children (Söderling 2009), xylitol use may be effective in altering MS colonization or virulence rather than by reduction of MS levels. Our study indicated that xylitol wipe use in children altered the stability of MS colonization but showed no impact on xylitol tolerance, acid production, and biofilm formation of MS (Zhan, Featherstone, et al. 2012). Since mutacin activity may affect MS transmission (Zhan, Tan, et al. 2012), we also investigated the mutacin gene distribution in MS through high-throughput Illumina sequencing of the MS isolated from the xylitol wipe study. Fifteen biosynthetic gene clusters were identified that account for the majority of variation in the MS genome and may have mutacin activity. However, no statistically significant differences were found in mutacin gene distribution between MS isolated from the xylitol versus the placebo groups (unpublished data). Because the etiology of dental caries is microbiome dysbiosis, future studies on anticaries mechanisms against bacteria transmission should include other cariogenic and commensal bacteria, microbiome changes, and bacterial virulence factors in both mothers and children. There are no studies that investigate the potential of erythritol to prevent bacterial transmission and caries formation.
In addition, there is new insight into the linkage of sugar consumption with systemic noninfectious diseases, including obesity, diabetes, hypertension, and cardiovascular diseases (World Health Organization 2015). As a main source for natural sugar substitutes, polyols, especially xylitol and erythritol use, hold a unique position to have not only oral benefits but also overall health benefits with respect to the noninfectious diseases listed above. Future dental studies should also include measurements for these systemic diseases.
Conclusion
Dental caries is a disease that results from microbiome dysbiosis with involvement of multiple cariogenic species, including MS, lactobacilli, and S. wiggsiae in the Actinomyces/Bifidobacterium family and several Actinomyces species that have the cariogenic traits of acid production and acid tolerance. It is hypothesized that selected putative cariogenic species may be involved in more aggressive dental caries while extended multiple species determinations will be warranted to determine the caries profile of the population and/or individuals under study. Therefore, anticaries treatment may be more logical in focusing on correcting the microbiome dysbiosis than eliminating a single pathological agent.
Sugar intake plays an important role, leading to microbiome dysbiosis, thereby determining the fate of caries development. The MS transmission involves multiple mechanisms and can have long-term impacts on the oral microbiome. Intervention in cariogenic bacteria transmission in early childhood may provide effective long-term caries prevention. Xylitol and erythritol may also serve as good candidates as part of anticaries regimens via their roles as sugar substitutes, oral microbiome modifiers, and agents to prevent cariogenic bacteria transmission with both oral and systemic benefits in early childhood. Additional studies are needed to further elucidate the mechanisms of action of xylitol and erythritol with respect to microbiome shifts and bacterial virulence in addition to cariogenic bacteria levels, as well as their benefits for overall health.
The anticaries agent C16G2, targeting the most studied cariogenic bacteria S. mutans, has shown good safety for short-term oral topical use and promising effects in reducing S. mutans in vitro and in vivo with the simultaneous promotion of oral commensal bacteria. Future study on its anticaries effect will need to include its long-term impact on the oral microbiome and effects on other important cariogenic bacteria.
Author Contributions
L. Zhan, contributed to conception, design, and data analysis, drafted and critically revised the manuscript. The author gave final approval and agrees to be accountable for all aspects of the work.
Acknowledgments
Xylitol and placebo wipes were provided free of charge from DR Products. Charles Hoover and Joanne Rahman have contributed to the major research activity for multigenerational transmission of mutans streptococci. Mohamed Abou Donia, Michael Fischbach, and Charles Le have been major contributors to the research activity for genomic sequencing and mutacin gene studies on mutans streptococci isolated from subjects in the xylitol wipe study. John D.B. Featherstone helped to edit the manuscript.
Footnotes
This research project was supported by the California Society of Pediatric Dentistry Foundation, Graduate Scientific Research Award from American Association of Pediatric Dentistry, Academic Senate Grant from the University of California San Francisco, and National Institutes of Health/National Institute of Dental and Craniofacial Research grant U54 DE019285.
The author declares no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Alaluusua S, Renkonen O. 1983. Streptococcus mutans establishment and dental caries experience in children from 2 to 4 years old. Scand J Dent Res. 91(6):453–457. [DOI] [PubMed] [Google Scholar]
- Caufield PW, Dasanayake AP, Li Y, Pan Y, Hsu J, Hardin JM. 2000. Natural history of streptococcus sanguinis in the oral cavity of infants: evidence for a discrete window of infectivity. Infect Immun. 68(7):4018–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffee BW, Featherstone JD, Gansky SA, Cheng J, Zhan L. 2016. Caries risk assessment item importance: Risk designation and caries status in children under age 6. JDR Clin Trans Res. 1(2):131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cock P, Makinen K, Honkala E, Saag M, Kennepohl E, Eapen A. 2016. Erythritol is more effective than xylitol and sorbitol in managing oral health endpoints. Int J Dent. 2016:9868421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doméjean S, Zhan L, DenBesten PK, Stamper J, Boyce WT, Featherstone JD. 2010. Horizontal transmission of mutans streptococci in children. J Dent Res. 89(1):51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R, Sullivan R, Shi W. 2012. Targeted antimicrobial treatment to re-establish a healthy microbial flora for long-term protection. Adv Dent Res. 24(2):94–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelsson IM. 2001. Mutans streptococci—in families and on tooth sites: studies on the distribution, acquisition and persistence using DNA fingerprinting. Swed Dent J Suppl. 2001(148):1–66. [PubMed] [Google Scholar]
- Featherstone JD, White JM, Hoover CI, Rapozo-Hilo M, Weintraub JA, Wilson RS, Zhan L, Gansky SA. 2012. A randomized clinical trial of anticaries therapies targeted according to risk assessment (caries management by risk assessment). Caries Res. 46(2):118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, McLean JS, Yang Y, Eckert R, Kaplan CW, Kyme P, Sheikh O, Varnum B, Lux R, Shi W, et al. 2015. Precision-guided antimicrobial peptide as a targeted modulator of human microbial ecology. Proc Natl Acad Sci U S A. 112(24):7569–7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgerson PL, Sjöström I, Stecksén-Blicks C, Twetman S. 2007. Dental plaque formation and salivary mutans streptococci in schoolchildren after use of xylitol-containing chewing gum. Int J Paediatr Dent. 17(2):79–85. [DOI] [PubMed] [Google Scholar]
- Hughes CV, Dahlan M, Papadopolou E, Loo CY, Pradhan NS, Lu SC, Mathney JM, Bravoco A, Kent RL, Jr, Tanner AC. 2012. Aciduric microbiota and mutans streptococci in severe and recurrent severe early childhood caries. Pediatr Dent. 34(2):e16–e23. [PMC free article] [PubMed] [Google Scholar]
- Janakiram C, Deepan Kumar CV, Joseph J. 2017. Xylitol in preventing dental caries: a systematic review and meta-analyses. J Nat Sci Biol Med. 8(1):16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo G, Zhan L, Hoover C, Featherstone JD. 2005. Cariogenic virulence characteristics of mutans streptococci isolated from caries-active and caries-free adults. J Calif Dent Assoc. 33(12):973–980. [PubMed] [Google Scholar]
- Köhler B, Birkhed D, Olsson S. 1995. Acid production by human strains of Streptococcus mutans and Streptococcus sobrinus. Caries Res. 29(5):402–406. [DOI] [PubMed] [Google Scholar]
- Kressirer CA, Smith DJ, King WF, Dobeck JM, Starr JK, Tanner AC. 2017. Scardovia wiggsiae and its potential role as a caries pathogen. J Oral Biosci. 59(3):135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitala ML, Alanen P, Isokangas P, Söderling E, Pienihakkinen K. 2013. Long-term effects of maternal prevention on children’s dental decay and need for restorative treatment. Community Dent Oral Epidemiol. 41(6):534–540. [DOI] [PubMed] [Google Scholar]
- Leverett DH, Proskin HM, Featherstone JD, Adair SM, Eisenberg AD, Mundorff-Shrestha SA, Shields CP, Shaffer CL, Billings RJ. 1993. Caries risk assessment in a longitudinal discrimination study. J Dent Res. 72(2):538–543. [DOI] [PubMed] [Google Scholar]
- Li Y, Caufield PW. 1995. The fidelity of initial acquisition of mutans streptococci by infants from their mothers. J Dent Res. 74(2):681–685. [DOI] [PubMed] [Google Scholar]
- Lindquist B, Emilson CG. 2004. Colonization of Streptococcus mutans and Streptococcus sobrinus genotypes and caries development in children to mothers harboring both species. Caries Res. 38(2):95–103. [DOI] [PubMed] [Google Scholar]
- Loesche WJ. 1986. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 50(4):353–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickenautsch S, Leal SC, Yengopal V, Bezerra AC, Cruvinel V. 2007. Sugar-free chewing gum and dental caries: a systematic review. J Appl Oral Sci. 15(2):83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgrom P, Söderling EM, Nelson S, Chi DL, Nakai Y. 2012. Clinical evidence for polyol efficacy. Adv Dent Res. 24(2):112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SC, Ruby JD, Moser S, Momeni S, Smith A, Osgood R, Litaker M, Childers N. 2009. Maternal transmission of mutans streptococci in severe-early childhood caries. Pediatr Dent. 31(3):193–201. [PMC free article] [PubMed] [Google Scholar]
- Simón-Soro A, Belda-Ferre P, Cabrera-Rubio R, Alcaraz LD, Mira A. 2013. A tissue-dependent hypothesis of dental caries. Caries Res. 47(6):591–600. [DOI] [PubMed] [Google Scholar]
- Söderling EM. 2009. Xylitol, mutans streptococci, and dental plaque. Adv Dent Res. 21(1):74–78. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Nyvad B. 2011. The role of bacteria in the caries process: ecological perspectives. J Dent Res. 90(3):294–303. [DOI] [PubMed] [Google Scholar]
- Tanner AC. 2015. Anaerobic culture to detect periodontal and caries pathogens. J Oral Biosci. 57(1):18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner AC, Kent RL, Jr, Holgerson PL, Hughes CV, Loo CY, Kanasi E, Chalmers NI, Johansson I. 2011. Microbiota of severe early childhood caries before and after therapy. J Dent Res. 90(11):1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner AC, Kressirer CA, Faller LL. 2016. Understanding caries from the oral microbiome perspective. J Calif Dent Assoc. 44(7):437–446. [PubMed] [Google Scholar]
- Tanner AC, Mathney JM, Kent RL, Chalmers NI, Hughes CV, Loo CY, Pradhan N, Kanasi E, Hwang J, Dahlan MA, et al. 2011. Cultivable anaerobic microbiota of severe early childhood caries. J Clin Microbiol. 49(4):1464–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner AC, Sonis AL, Lif Holgerson P, Starr JR, Nunez Y, Kressirer CA, Paster BJ, Johansson I. 2012. White-spot lesions and gingivitis microbiotas in orthodontic patients. J Dent Res. 91(9):853–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer JM, Livingston J, Thompson AM. 2001. The microbiology of primary dental caries in humans. J Dent Educ. 65(10):1028–1037. [PubMed] [Google Scholar]
- Wan AK, Seow WK, Purdie DM, Bird PS, Walsh LJ, Tudehope DI. 2003. A longitudinal study of Streptococcus mutans colonization in infants after tooth eruption. J Dent Res. 82(7):504–508. [DOI] [PubMed] [Google Scholar]
- World Health Organization. 2015. Guideline: sugars intake for adults and children. Geneva (Switzerland): World Health Organization. Available from: www.Ncbi.Nlm.Nih.Gov/books/nbk285537 [PubMed]
- Young DA, Featherstone JD. 2013. Caries management by risk assessment. Community Dent Oral Epidemiol. 41(1):e53–e63. [DOI] [PubMed] [Google Scholar]
- Zhan L, Cheng J, Chang P, Ngo M, Denbesten PK, Hoover CI, Featherstone JD. 2012. Effects of xylitol wipes on cariogenic bacteria and caries in young children. J Dent Res. 91(7 Suppl):85S–90S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan L, Featherstone JD, Gansky SA, Hoover CI, Fujino T, Berkowitz RJ, Den Besten PK. 2006. Antibacterial treatment needed for severe early childhood caries. J Public Health Dent. 66(3):174–179. [DOI] [PubMed] [Google Scholar]
- Zhan L, Featherstone JD, Lo J, Krupansky C, Hoang N, DenBesten P, Huynh T. 2012. Clinical efficacy and effects of xylitol wipes on bacterial virulence. Adv Dent Res. 24(2):117–122. [DOI] [PubMed] [Google Scholar]
- Zhan L, Tan S, Den Besten P, Featherstone JD, Hoover CI. 2012. Factors related to maternal transmission of mutans streptococci in high-risk children-pilot study. Pediatr Dent. 34(4):e86–e91. [PubMed] [Google Scholar]