Abstract
In this review, we summarize the most important recent developments in the treatment of amyotrophic lateral sclerosis (ALS). In terms of disease-modifying treatment options, several drugs such as dexpramipexole, pioglitazone, lithium, and many others have been tested in large multicenter trials, albeit with disappointing results. Therefore, riluzole remains the only directly disease-modifying drug. In addition, we discuss antisense oligonucleotides (ASOs) as a new and potentially causal treatment option.
Progress in symptomatic treatments has been more important. Nutrition and ventilation are now an important focus of ALS therapy. Several studies have firmly established that noninvasive ventilation improves patients’ quality of life and prolongs survival. On the other hand, there is still no consensus regarding best nutritional management, but big multicenter trials addressing this issue are currently ongoing. Evidence regarding secondary symptoms like spasticity, muscle cramps or sialorrhea remains generally scarce, but some new insights will also be discussed. Growing evidence suggests that multidisciplinary care in specialized clinics improves survival.
Keywords: amyotrophic lateral sclerosis, antisense nucleotides, nutrition, riluzole, symptomatic therapy, ventilation
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal motor neurone disease (MND) characterized by symptoms of degeneration of the upper motor neurons (UMNs) in the motor cortex as well as the lower motor neurons (LMNs) in the spinal cord and brain stem. This leads to progressive paresis of all voluntarily innervated muscles and therefore affects mobility, communication, swallowing, and breathing. Death typically occurs after a mean survival of 2–4 years,1 usually due to respiratory failure. Hereditary (‘familial’, 10% of patients) and sporadic forms (90% of patients) exist, and several gene mutations (SOD1, C9ORF, FUS, TDP-43, and others) are implicated in the pathogenesis of ALS. The mean age of onset is 58–63 years in sporadic ALS and 43–52 years in familial ALS.2
Although motor symptoms clearly dominate the clinical picture, ALS is a multisystem degeneration, and nonmotor symptoms like cognitive impairment, psychiatric disorders, extrapyramidal, sensory, and autonomic symptoms are increasingly recognized. Overall, 5–10% of ALS patients develop frontotemporal dementia (FTD).3 As neuropathological correlate, pTDP-43, the hyperphosphorylated and ubiquinated form of a RNA- and DNA-binding protein, accumulates in neurons and consecutively spreads along the brain and spinal cord of ALS patients.4
According to the recently revised and simplified El-Escorial criteria,5 formal diagnosis of ALS requires progressive UMN and LMN symptoms in one body region (bulbar, cervical, thoracic, lumbosacral) or clinical or electromyographic LMN symptoms in two body regions. Restricted endophenotypes of ALS include primary lateral sclerosis (PLS) with exclusive UMN involvement and progressive muscular atrophy with exclusive LMN involvement.
Since ALS patients suffer from a wide range of debilitating symptoms, therapy management is a complex multidisciplinary challenge. As the disease progresses, new problems emerge and therapeutic strategies have to be adjusted dynamically. Also, coping with the diagnosis is a prolonged process in ALS. Patient’s attitudes towards invasive and life-prolonging measures are often ambivalent and can change over the course of the disease. Adequate, graduated communication of diagnosis and its implications, monitoring of disease progression, management of symptoms, aid advice, psychological and social support including end-of-life decisions are essential aspects of ALS care and are best performed by a multidisciplinary team with expertise in ALS.6 Of note, attendance in specialized multidisciplinary clinics appears to improve quality of life and survival.7
Disease-modifying therapy options for most ALS patients today are still restricted to riluzole, which will be discussed in detail in the first section of this review. Over the past years, several placebo-controlled multicenter trials which investigated different drugs in order to improve survival were unsuccessful. Tested substances include dexpramipexole,8 pioglitazone,9 and lithium.10 In the lithium study, the primary endpoint was the time to an event, defined as a decrease of at least six points of the revised ALS functional rating scale score (ALSFRS-R) or death. In the dexpramipexole trial, the primary endpoint was the combined assessment of function and survival score, based on changes in ALSFRS-R and time to death. The pioglitazone trial used survival as primary endpoint.
Recently, a Japanese study with edaravone showed a beneficial effect for a subgroup of early ALS patients, which is discussed in the second section. Furthermore, antisense nucleotides might possibly offer an interesting future treatment option at least for a subgroup of familial ALS patients and are discussed in the third section.
The subsequent sections cover symptomatic treatment options, including nutrition and ventilation (including noninvasive ventilation, invasive ventilation, and diaphragm pacing) as well as common aggravating symptoms like spasticity, muscle cramps, pain, and sialorrhea. The level of evidence for symptomatic treatment measures in ALS is generally low, therefore international guidelines partly rely on case series and expert opinions.
In order to provide reliable and up to date recommendations, we performed an extensive literature search of all available medical reference systems, including MEDLINE (since January 1966), Cochrane Central / Cochrane Neuromuscular Disease Group Specialized Register, Cochrane Library, EMBASE (since January 1980), AMED (since January 1985), CINAHL plus (since January 1938), LILACS (since January 1982), OVID HealthSTAR (since January 1975), clinicaltrials.gov (since January 1997), and International Clinical Trials Search Portal (since November 2004). We considered all clinical trials up to the date of our last search (4 May 2017) addressing the issue of symptomatic therapy options for ALS without any further limitation, but focused on randomized controlled trials (RCTs). We also considered international guidelines including the European Federation of Neurological Societies (EFNS), the American Academy of Neurology (AAN), the German Society of Neurology (Deutsche Gesellschaft für Neurologie; DGN), and the UK National Institute for Health and Care Excellence (NICE).
Riluzole
In Europe to date, riluzole remains the only drug approved for the treatment of ALS. Different action mechanisms of riluzole are discussed: via its influence on glutamate metabolism, riluzole increases extracellular glutamate uptake and inhibits glutamate release from presynaptic terminals. The drug also interferes with N-methyl-D-aspartate receptor mediated responses and stabilizes inactivated state of voltage-dependent sodium channels. Riluzole prolongs median tracheostoma-free survival by about 6 months (18.3 months versus 12.4 months;11) and increases survival rate compared with controls.12,13 A more recent Cochrane review reports a median prolongation of survival of 3 months (14.8 versus 11.8 months).14 Despite the only moderate effect and relatively high costs, treatment with riluzole is recommended in the AAN, EFNS, and other neurological guidelines as it has a safe drug profile and is usually well tolerated.
The recommended dosage for riluzole is 100 mg per day in two single doses of 50 mg.14 Liver function should be monitored during the first months of treatment. Serum levels of transaminases should be controlled every 4 weeks during the first 3 months and once every 3 months during the further course of disease. Treatment should be discontinued if serum levels of transaminases exceed three-fold the normal value.14 In patients with initially elevated liver enzymes, transaminases should be controlled more frequently. Cases of marked neutropenia (absolute neutrophil count less than 500/mm3) are very rare. Patients should be instructed to inform their physician if they develop febrile illness, and white blood cell count should be performed in these cases. In patients treated with riluzole, interstitial lung diseases have been observed, some of them severe. Several cases were identified as hypersensitivity pneumonitis. If patients report dry cough, this should prompt further diagnostic steps. In case of findings suggestive of interstitial lung disease or hypersensitivity pneumonitis (e.g. bilateral diffuse lung opacities), riluzole treatment should be discontinued. Other side effects that have been observed in clinical trials include asthenia, nausea, headache, and hypertension. Recently, a liquid form of riluzole has been developed which can be used in patients who are unable to swallow solid tablets.15
Edaravone
In May 2017, the United States (US) Food and Drug Administration (US FDA) approved edaravone for the treatment of ALS in the US. Edaravone is an antioxidant which had originally been used for the treatment of stroke patients in Japan. Its mode of action in ALS is largely unknown. In the first phase III study, edaravone did not show a significant effect compared with placebo, but post-hoc analysis revealed that a subgroup of patients in an early stage might benefit from the drug. Therefore, a second placebo-controlled multicenter phase III study that focused on this subgroup was conducted in Japan in 137 patients.16 Only patients with ALS grade 1 or 2 in the Japan ALS Severity Classification, scores of at least 2 points in all 12 items of the ALS-FRS-R, a forced vital capacity of 80% or more, and a disease duration of 2 years of less were included. Eligible patients also had a decrease of 1–4 points in the ALS-FRS-R score during a 12-week observation period before randomization, reflecting a rather rapid course of disease.
Patients received 60 mg intravenous edaravone or placebo for 6 cycles (4 weeks per cycle with 2 weeks on, 2 weeks off) in 24 weeks. In the first cycle, the drug was administered daily for 14 days, followed by a 2-week drug-free period. In the second cycle and thereafter, it was administered for 10 days within a 14-day period, followed by a 2-week drug-free period. There decline of ALS-FRS-R was −5.01 [standard error (SE) 0.64] in the edaravone group and −7.50, (SE 0.66) in the placebo group. The difference between both groups (2.49, SE 0.76) was highly significant [confidence interval (CI) 0.99–3.98, p = 0.0013]. Frequency of adverse events was similar in both groups (84%), and serious adverse events were more frequent in the placebo group (24%) than in the edaravone group (16%). The most common adverse reactions were bruising and gait disturbance.
In summary, the study showed a beneficial effect of edaravone in a well-defined group of early ALS patients. Of note, although the authors emphasize that there was no indication that edaravone was effective in a wider population of ALS patients, the drug was approved for all ALS patients in the US without any restrictions. To date, edaravone has not been approved in Europe. Although the drug is available via international pharmacies, its use in clinical practice is currently restricted due to its high costs and the lack of an oral administration form. We believe that further studies are needed in order to evaluate different dosages and the effect on survival.
Antisense nucleotides as a potential causal treatment option
The molecular mechanisms underlying ALS pathogenesis are still not fully understood. Studies in both animal models and humans show different alterations on multiple levels, such as increased glutamate-mediated excitotoxicity, increased apoptosis, defective axonal transport, oxidative stress, mitochondrial impairment, sustained unregulated immune responses and accumulation of misfolded proteins.17
Aggregation of toxic proteins, like hyperphosphorylated tau protein in Alzheimer’s dementia or Lewy bodies in Parkinson’s diseases, is one possible pathogenic mechanism in ALS and other neurodegenerative diseases18 and reduction of toxic protein levels has thus been discussed as a therapeutic approach.
Around 5–10% of all ALS cases are caused by pathogenic gene mutations, the most common being a mutation in an open reading frame on chromosome 9 (C9ORF72) which accounts for around 35% of familial ALS cases, followed by a mutation of the gene coding for superoxide dismutase 1 (SOD1), responsible for about 15% of familial cases.19
The only known physiological function of the SOD1 protein is the catalysis of a hyperoxide (or superoxide) anion and formation of SOD1 homodimers. The exact nature of the toxicity of SOD1 remains incompletely understood, but accumulation and toxic gain-of-function of the misfolded protein are likely involved, making this a plausible target for gene-silencing therapies. Interestingly, misfolded proteins can also be found in protein aggregates of a large proportion of sporadic ALS cases, indicating a more widespread role for their abnormal localization in ALS pathogenesis and pointing to a possible effect of antisense nucleotides (ASOs) also in nonmutation carriers.20
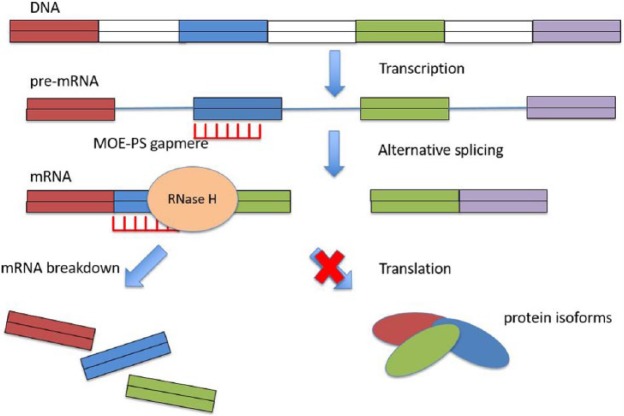
Short, synthetic oligonucleotides (15–25 nucleotides) bind by Watson–Crick hybridization to target mRNA in a sequence-specific manner.21 The mRNA then undergoes catalytic degradation by endogenous RNase H in the nucleus.22 Different modes of action have been described in different ASOs. In the case of ASOs targeting SOD1, application of the drug leads to reduced mRNA levels of the SOD1 transcript23 (Figure 1). ASOs targeting SOD1 were shown to be effective and well tolerated in the mouse model as well as in nonhuman primates. In these models, after application of ASOs, levels of misfolded protein were significantly lower in the brain and spinal fluid.24
Figure 1.
Mode of action of ASOs in patients with SOD1 mutations.
ASO, antisense oligonucleotide.
A first phase I trial in humans was conducted in 2013.25 While the drug was well tolerated with no serious adverse events, the main hurdle for the routine clinical approach is the fact that application requires intrathecal injection to reach the cerebrospinal fluid. Accordingly, most adverse events in the first trial in humans were related to the lumbar puncture procedure. A combination of ASOs with potent vectors that pass the blood–brain barrier are in development, but have not been tested in humans to date.26 At the moment, a large multicenter phase I trial is recruiting SOD1-patients in the US, Canada, and Europe [ClinicalTrials.gov Identifier: NCT02623699]. Primary outcome measures include abnormalities in the neurological and physical examination as well as maximum concentration levels and elimination half-time of the administered ASOs in the plasma and spinal fluid of patients. It is still too early to provide any evidence on the effectiveness of ASO treatment in SOD1 and possibly also other single-gene-related and sporadic ALS cases.
ASOs are emerging as a promising therapeutic approach also for several other neurodegenerative diseases. In spinal muscular atrophy (SMA), a recent phase I study showed that intrathecal application of ASOs was well tolerated, and children treated with ASOs showed increased motor function, evaluated by Hammersmith Functional Motor Scale Expanded scores after 3 months (+3.1 points; p = 0.016) and 9–14 months (+5.8 points; p = 0.008).27 Therefore the drug has just been approved for SMA patients in the US and in Europe.
Nutrition
Many ALS patients undergo rapid weight loss, which is a negative prognostic factor. Therefore, nutrition is an important issue in the management of ALS. Malnourished patients have a 7.7-fold increased risk of death28 and a 30% increased risk of death per 5% of weight loss.29 The underlying mechanisms of weight loss in ALS remain incompletely understood. Multiple causes like dysphagia and muscle wasting are being discussed, but lately most attention is focused on intrinsically altered metabolism. ALS patients show increased resting energy expenditure.30 This precedes motor symptoms and remains stable over the course of disease. The pioglitazone trial9 showed that ALS patients in the treatment arm did not gain body weight as expected, suggesting a dysfunction of the hypothalamus.31
Fat metabolism has been discussed as a possible prognostic factor as well. Studies in large French and German cohorts show an increased survival by 12 months for patients with an increased low density lipoprotein/high density lipoprotein ratio32 and an increased survival by 21.6 months for patients with hypertriglyceridemia.33 However, in an Italian and an US study, hyperlipidemia was not associated with increased survival.34,35 Cultural eating habits might contribute to these discrepancies. The question whether hyperlipidemia is an independent prognostic factor remains unresolved to date.
Of note, one prospective cohort study with 164 ALS patients (32 with and 132 without statin medication) has shown a 63% accelerated decline in the ALS functional rating scale revised (ALS-FRS-R) for patients on statins.36
Considering the findings above, several recent studies focused on increasing body weight or serum cholesterol and triglycerides in ALS patients. In an animal model, high-caloric nutrition was associated with significant weight gain and increased survival.37 A small prospective study has proven that body weight stabilization with high-caloric, either high-fat or high-carbohydrate nutrition is possible in ALS patients.38 Retrospective data suggest a positive effect on survival as well.39 However, prospective controlled trials with sufficient power are currently running to improve the evidence base. In a placebo-controlled multicenter German study, patients are treated with a high-caloric, high-lipid drink in addition to their normal intake [ClinicalTrials.gov Identifier: NCT02306590], while a French study investigates different patterns and doses of fat- and protein-enriched diets dependent on the extent of weight loss [ClinicalTrials.gov Identifier: NCT02152449].
Currently, the EFNS guidelines40 recommend to control nutritional status and body weight regularly and to discuss the timing of tube feeding on an individual basis. The findings described above suggest that ALS patients who suffer from weight loss should be put on a high-caloric diet or food supplements in order to stabilize body weight. Due to current lack of evidence, no recommendation can be made regarding the share of fat and carbohydrates. Indication for lipid lowering drugs like statins should be checked carefully, and an individual evaluation of risk versus benefit should be made for each patient.
Several studies compare different methods of tube feeding in ALS. In general, the purpose of nutrition via gastrostomy is to stabilize body weight and to reduce risk of aspiration in patients with dysphagia.40 The most common method is percutaneous endoscopic gastrostomy (PEG), followed by radiologically inserted gastrostomy (RIG) and per-oral image-guided gastrostomy (PIG). PEG refers to a procedure in which a tube is passed into the patient’s stomach through the abdominal wall, while a lighted endoscope is passed orally into the stomach to assist with placement and fixation of the tube. In contrast, during RIG, the percutaneous gastrostomy is guided by X-ray. PIG is an image-guided hybrid technique in which the tube is placed through the mouth. A recent meta-analysis showed no difference regarding survival between all three methods,41 and a large, longitudinal, prospective cohort study in the UK found equal safety regarding survival and procedural complications.42 Therefore it is possible to choose the method according to local expertise.
Regarding optimal timing of gastrostomy, some retrospective studies report an increased peri-procedural risk for patients with vital capacity <50%.6 Therefore it has been suggested to perform gastrostomy early as soon as progressive weight loss or risk of aspiration occur. However, a recent prospective observational study in a large cohort of patients43 showed that PEG was safe even in patients with far advanced disease and low vital capacity. Several studies support the view that in those patients noninvasive ventilation should be established prior to PEG and used during PEG insertion using special masks.44 The EFNS guidelines recommend early gastrostomy and mention bulbar symptoms, malnutrition (weight loss >10%), respiratory function, and patient’s general condition as factors which have to be considered for timing.40 Also, the patient’s attitude towards tube feeding has to be taken into account. Some patients reject invasive or life-prolonging measures in general, or they hesitate to give up the pleasure of eating. Therefore, gastrostomy should be discussed openly at an early stage and revisited regularly, and the patient’s individual preferences should be respected. In this context, the presence of cognitive impairment and the patient’s ability to make decisions have to be considered. A recent study in a large cohort of patients found that mild and moderate cognitive and behavioral impairments did not influence medical decisions in ALS45 as opposed to patients with manifest FTD.
Similar to oral nutrition, there is growing evidence that high-caloric feeding might be beneficial for tube feeding as well. A recent prospective trial showed an increased survival for hypercaloric compared with an isocaloric diet46 in a small number of patients, and an observational study in a larger sample found a benefit for patients receiving at least 1500 kcal daily.43
Periprocedural administration of single dose of antibiosis reduces the risk of peristomal infections in general,47 and in ALS specifically it has been shown that single-shot antibiosis with cefuroxime was associated with lower C-reactive protein values43 compared with patients without treatment with antibiotics.
Noninvasive ventilation
During the disease course, most patients develop chronic respiratory insufficiency due to progressive weakness of the diaphragm and auxiliary respiratory muscles. Early symptoms include signs of hypercapnia such as daytime fatigue, sleep disturbance, cognitive impairment, and depression, while dyspnea and orthopnea usually occur later. Respiratory disorders usually develop slowly in ALS; therefore, acute worsening should lead to diagnostic measures in order to exclude complications like atelectasis, pneumonia, or pulmonary embolism.
Noninvasive ventilation (NIV) refers to the administration of ventilatory support through the upper airways without using invasive artificial airways like endotracheal tubes or tracheostomy. It is performed by using a compact breathing device with a nasal or full-face mask. It is easy to handle and can be used at home, initially usually at night. Because of its flexible and noninvasive nature, it is usually well accepted. NIV uses room air, since administration of pure oxygen reduces the respiratory drive in ALS patients and might induce carbon dioxide narcosis.
NIV has become an important cornerstone of ALS therapy and should be initiated as soon as clinical symptoms appear. Additional diagnostic measures such as forced vital capacity (FVC), sniff nasal inspiratory pressure (SNIP), maximal inspiratory pressure, blood gas analysis, oximetry, and capnometry can be used to justify an earlier initiation of NIV although there is no evidence or standardized procedure. The EFNS guidelines recommend to initiate NIV when at least one of the aforementioned clinical symptoms or one of the following criteria is present: FVC < 80%, SNIP < 40 cm H2O, significant nocturnal desaturation, pCO2 > 45 mmHg (morning blood gas).
Several studies have proven a life-prolonging effect of NIV although size remains difficult to gauge due to lack of placebo-controlled studies. The only available RCT (N = 41) has shown a prolonged survival of about 7 months for NIV patients48 as well as an improved quality of life as measured by mental component summary and sleep apnea quality of life index symptoms domain (sym). A recent large retrospective study (N = 929) found a prolonged survival effect of 13 months.49
NIV also improves blood gas parameters and respiratory symptoms. Recent polysomnographic studies have demonstrated improvement of oxygen saturation, apnea–hypopnea index, and transcutaneous carbon dioxide tension,50 and also an improvement of sleep quality, daytime fatigue, depression,51 and quality of life.52 Although direct correlations between respiratory symptoms and blood gas parameters have not been reported in the aforementioned studies, improvement of blood gas parameters and quality of life indicators both remained stable over an observation period of 10 months.51
Although they still might benefit from NIV, patients with neurobehavioral abnormalities have reduced compliance and survival.53 Adaptation to NIV can be difficult, therefore multidisciplinary treatment in a hospital setting is considered beneficial.54 Patients with pronounced bulbar involvement remain a challenge since salivation and viscous airway mucus hinder mask ventilation and reduce compliance as well as prognosis,55 therefore optimal secretion management (see section on sialorrhea) is crucial for those patients. Although the evidence level is low, in our experience mechanical insufflation-exsufflation devices (‘cough assist’) can be used in order to free the upper airways from rough mucus and therefore facilitate the subsequent ventilation session. These devices are generally well tolerated and improve respiratory outcome parameters in ALS.56 It is important to overcome the above-mentioned issues, since recent data suggest that, in contrast with previous studies, bulbar patients do benefit from NIV as well.49
Further barriers for NIV usage include general reservations towards the technology, concerns about sleep disturbance, the uncomfortable sensation of pressure and pulsing, dry mouth, and mask design issues.57 Most of these issues can be overcome with time, therefore information about long-term benefits and continued support from respiratory specialists are of great importance for compliance.
Some patients are prone to panic attacks under mask ventilation and this can be managed with opioids or benzodiazepines, but empirical data are missing. These measures have to be adopted with great care and by experienced physicians only as they reduce the patient’s respiratory drive and therefore promote carbon dioxide retention. EFNS guidelines suggest to treat anxiety with sublingual lorazepam or tablets in a dosage of 0.5 mg two or three times daily.40
There are very few studies investigating which time frames and technical NIV parameters should be used. The most common practical approach according to NICE guidelines is to perform initial acclimatization during the day when the patient is awake. Regular treatment is usually started at night, before and during sleep. Further disease progression usually mandates an extension of NIV periods during the day until 24 h are reached in some patients. Regular monitoring of respiratory symptoms and respiratory function tests as described above should be used to determine the extension of ventilation times.
There was one study that showed no difference between pressure-controlled and volume-controlled ventilation with regard to survival.58 Since other parameters have not been investigated systematically to date, they are usually adjusted individually to reflect patient’s physiological and most comfortable breathing pattern and therefore maximize compliance.
Invasive ventilation
There is no standardized guideline regarding initiation and use of invasive ventilation (IV) via tracheostomy. Due to its invasive nature and extreme demands of care, it is usually regarded as a late option for patients who cannot be stabilized by NIV or who do not tolerate it. It might also be considered in order to secure proper secret management in some cases.
Patients have to be informed in depth about consequences, and their psychosocial background has to be considered. Survival can be prolonged considerably by IV59 which implies that patients experience far advanced stages of disease up to complete paralysis and locked-in syndrome. Many patients reject IV because they do not feel that quality of life is acceptable under such conditions.
End-of-life management
Regarding the terminal phase of ALS it is important to inform patients that death usually occurs peacefully as a result of carbon dioxide narcosis and that fear of suffocation is unfounded.60 Symptomatic treatment with opioids, benzodiazepines, and oxygen can sometimes be required to avoid or reduce dyspnea and anxiety,40 but this is usually manageable even at home. While pure oxygen can be useful in terminal phase as palliative treatment, it is not appropriate for long-term treatment of chronic hypoventilation in ALS because it can produce respiratory depression. Patients who decide to discontinue NIV or IV treatment should be supported by experienced healthcare specialists and consequences should be discussed openly.
Advance directives can help to avoid unwanted situations and should be as specific as possible. Patients should be informed in depth about all aspects of life-prolonging measures before preparing an advance directive. The advance directive should include the patient’s attitude towards NIV, IV, and PEG specifically and under which circumstances life-prolonging measures should be stopped. Patients should be informed about the legal situation regarding withdrawal from life-prolonging measures and assistance in formulating the advance directive should be offered.40
Diaphragm pacing
Based on unpublished data of an US trial, electrical diaphragm stimulation (diaphragm pacing system, DPS) has been approved by the US FDA for ALS. DPS was supposed to have a life-prolonging effect, and it has been shown to improve quality of sleep.61 However, a recent British study showed that patients with NIV and DPS had shorter survival (−11.5 months, 95% CI 8.3–13.6 months) than patients with NIV alone.62 More recently, this result was complemented by a French study63 which showed that DPS did not delay the need of NIV as compared with sham stimulation and was associated with decreased survival. Median noninvasive ventilation-free survival was 6.0 months (95% CI 3.6–8.7 months) in the active stimulation group versus 8.8 months (4.2–not reached) in the control group [hazard ratio 1.96 (95% CI 1.08–3.56), p = 0.02]. Furthermore, serious adverse events related to the procedure like capnothorax, pneumothorax, acute respiratory failure, and venous thromboembolism, were very frequent (>50% of patients). Therefore, it has to be concluded that DPS should not be considered as therapy option in ALS anymore.
Physiotherapy
Physiotherapy (PT) is an established symptomatic treatment in ALS serving alleviation and compensation of motor deficits. However, due to the progressive nature of the disease improvement of muscle weakness is not a realistic therapy goal. Therefore, objectives of PT in ALS are preservation and strengthening of residual motor function and prevention of inactivity-related atrophy, contractions, lymphatic oedema, thrombosis, and musculoskeletal pain. The assumption is that PT has an impact on nonmotor dimensions of quality of life like patient’s perception of physical integrity, participation in life, and sleep routines.
Overall, three RCTs have proposed positive effects of moderate exercise programs on quality of life, fatigue, pain, and motor function but all of them are underpowered and suffer from limited outcome parameters, resulting in inconsistent conclusions. Drory and colleagues64 found a trend towards reduced deterioration in motor function, fatigue, quality of life, and pain after 6 months for N = 25 patients who received moderate daily exercise program, but this was not statistically different. Bello-Haas and colleagues65 found positive effects of daily stretching and resistance exercises on motor function and quality of life in N = 27 patients after 6 months. Lunetta and colleagues66 found no effect of three different methods of active exercise programs in N = 60 patients on survival or quality of life after 6 months, but proposed a positive effect on motor function. There are no data available for later stages of disease or longer treatment periods. As a consequence, a recent Cochrane review67 concluded that there was ‘a complete lack of randomized or quasi-randomized clinical trials’.
Of note, high-intensity endurance training was found to hasten death in an ALS mouse model.68 Considering that phrenic nerve stimulation is associated with a much shorter survival,62 overstimulation of nerval structures might be harmful in ALS. For those reasons, randomized controlled studies are needed to clarify the benefit of different methods and intensities of physiotherapy in ALS.
Spasticity
Increased muscle tone is a common phenomenon in ALS due to lesions of corticoefferent motor fibers. It can cause rigid/spastic stiffness in already weakened muscles and therefore further aggravate difficulties with mobility and daily activities. Furthermore, it can cause pain and secondary pathology of the musculoskeletal system like subluxations and contractures. Therefore, adequate treatment of increased muscle tone is of great importance.
Physiotherapy is generally regarded as the most important and effective therapy, but the evidence level is generally low as described above. The EFNS guidelines also mention that hydrotherapy and cryotherapy should be considered. RCTs are also missing for treatment with drugs, although many different compounds like baclofen, tizanidine, benzodiazepines, dantrolene, gabapentin, or memantine are used in clinical practice. In general, most anti-spastic drugs might cause fatigue and nausea and therefore have to be increased very carefully to ensure compliance. There are several reports in the literature about anti-spastic and possible other beneficial effects of cannabis in ALS,69 but there are no systematic approaches.
Since ALS is nowadays regarded a multisystem degeneration and might involve the extrapyramidal system, rigidity might contribute to increased muscle tone in some patients.70 In our experience dopaminergic drugs can be effective in a subset of patients, in particular patients with more preserved anterior horn cell function (PLS), although again systematic clinical data are completely missing.
When management with these drugs is not sufficient, more invasive treatment options can be considered in carefully selected patients. Vázquez-Costa and colleagues71 described a clinical benefit (measured by a 10 m walk test) in 7/7 patients and subjective improvement in 6/7 patients with moderate to severe spasticity treated with botulinumtoxin A.
In summary, due to low evidence of all available treatment options, clinical practice largely depends on personal experiences and preferences rather than systematic data.
Cramps
Cramps are painful, involuntary muscle contractions which are common in ALS. Just like the treatment of spasticity, treatment is largely empirical and not backed up by systematic data. A Cochrane Review of 201272 identified 20 studies with different drugs (vitamin E, baclofen, L-threonine, xaliproden, indinavir, memantine), which all failed to show favorable effects, but many studies were underpowered. The EFNS guidelines recommend levetiracetam,40 while the NICE guidelines mention quinine as first-line treatment, baclofen as second-line, and tizanidine, dantrolene, or gabapentin as third-line. In clinical practice, many other substances like magnesium, benzodiazepines, carbamazepine, or cannabis have been tried.
However, recently a double-blind, placebo-controlled study in 60 patients with mexiletine showed significant reduction of cramp frequency and intensity in a dosage of 300 mg/d.73 A higher dosage (900 mg/d) led to frequent discontinuation due to adverse effects and was therefore not recommended by the authors. The study also showed that mexiletine was safe with no serious adverse events in the group receiving 300 mg/d. This study provides the highest evidence for treatment of cramps in ALS available so far.
Surprisingly, physiotherapeutic interventions for the treatment of muscle cramps have been largely neglected by literature. Cessation of muscle cramps has been reported in a case series of three patients performing yoga.74 Further systematic studies are needed to assess different methods of physiotherapy and their effect on cramps in ALS.
Sialorrhea and bronchial secretions
Due to progressive weakness of pharyngeal muscles and reduced swallowing capability, sialorrhea is a common problem in ALS, increasing the risk of aspiration and preventing effective use of noninvasive ventilation. In clinical practice, a great variety of different methods are used to reduce sialorrhea, including botulinum toxin injections in salivary glands, anticholinergic drugs, and radiotherapy (RT), but the evidence level is generally low.
In one randomized, double-blind study in 20 patients, Jackson and colleagues75 found that after 2 weeks, 82% of patients who received 2500 units of botulinum toxin type B by bilateral injection in the parotid and submandibular glands reported a global impression of improvement compared with 38% of patients who received placebo (p < 0.05). In a prospective, randomized, double-blind, crossover study in 15 patients with ALS and 12 patients with Parkinson’s disease (PD), Guidubaldi and colleagues76 reported similar effects of bilateral, ultrasound-guided injections of botulinum toxin A (250 U) and B (2500 U) in the parotid and submandibular glands. However, they found that the effect of botulinumtoxin B had a shorter latency, lower costs, and comparable duration. Botulinum toxin A and B injections were well tolerated in all studies although patients should be informed that botulinum toxin sometimes worsens swallowing.77
Evidence for efficacy of radiotherapy for the treatment of sialorrhea is mostly based on case series. Guy and colleagues78 reported that 13/16 patients with different treatment protocols improved 1 month after radiation. Kasarskis and colleagues79 found beneficial effects in 10/10 patients treated with irradiation of a single parotid gland with 15 Gy in 3 fractions. Bourry and colleagues80 investigated 21 patients with different protocols. They found an overall positive response in 65% and an advantage for patients treated with electrons compared with photons and with higher doses (⩾16 Gy) compared with lower doses (<16 Gy). In the largest sample so far (N = 50), Assouline and colleagues81 applied RT to both submandibular glands and two-thirds of both parotid glands, comparing doses of 10 Gy in 2 fractions and 20 Gy in 4 fractions. They found significant improvement of Sialorrhea Scoring Scale in all patients and a better effect in the high-dose group. In a recent review comprising 10 studies and 216 patients with ALS or PD, Hawkey and colleagues82 reported an improvement in 81% of patients, short-term toxicity in 40%, and long-term toxicity in 12%. It has to be taken into account that radiotherapy can lead to irreversible dry mouth.
Regarding anticholinergic drugs, no RCTs are available. However, due to the invasive nature of the alternative therapies described above, anticholinergic drugs are usually applied first. Anticholinergic drugs may cause confusion, and some patients do not tolerate scopolamine patches due to allergic skin reactions. In a UK-wide survey, clinicians preferred scopolamine patches, amitriptyline, and carbocisteine for the treatment of sialorrhea. Botulinum toxin was used in 14/21 centers.83 The EFNS guidelines recommend amitriptyline, oral or transdermal hyoscine, or sublingual atropine drops, and botulinum toxin injections in refractory cases. Irradiation is mentioned as an option when pharmacological treatment fails.40 The NICE guidelines additionally mention glycopyrrolate as a first-line treatment option for patients with cognitive impairment because of fewer central nervous side effects.
There is hardly any evidence regarding management of viscous bronchial secretions. In practice, mucolytics like N-acetylcysteine or guaifenesin, beta-receptor antagonists like metoprolol or propranolol84 and bronchodilators like ipratropium and theophylline are used empirically. Additionally, manual or mechanical cough-assisting85 and suction devices proved beneficial in uncontrolled trials. Patients should also be informed about the importance of careful oral hygiene. The EFNS and NICE guidelines additionally recommend nebulizers with saline, anticholinergic bronchodilators or furosemide40 as well as humidification of room and ventilation air.
Pain
Pain is a common symptom in ALS86 and may be caused by many different factors like spasticity, muscle cramps, contractures, skin pressure, and neuropathic pain. Although pain is generally recognized as an important symptom in palliative care, systematic studies regarding therapy of pain in ALS are completely missing.
A Cochrane review of 201387 found no randomized studies or well-designed observational studies, possibly due to the fact that pain in ALS is still underappreciated or neglected entirely.
In the absence of evidence-based data, treatment of pain is usually based on the World Health Organization (WHO) analgesic ladder.88 This concept involves the prescription of nonsteroidal anti-inflammatory drugs (NSAIDs) for mild pain and the combination of NSAIDs and weak or strong opioids for moderate and severe pain respectively, possibly in combination with optional adjuvants like tricyclic antidepressants or anticonvulsants to treat neuropathic pain.
However, it is essential to carefully determine the etiology of pain in order to be able to derive causal treatment options whenever possible. Additionally, side effects and interactions of prescribed drugs have to be taken into account. Some analgesic drugs may provide additional benefits in ALS, such as alleviation of dyspnea and anxiety (opioids) or positive effects on salivation and depression (amitriptyline).
In our experience and in accordance with existing literature,89 the fear of respiratory depression in ALS patients treated with opioids is overestimated, and we are confident that low-dose opioid therapy is generally safe if combined with noninvasive ventilation.
Cognitive and behavioral symptoms
As mentioned above, the clinical spectrum of ALS and FTD is overlapping, and the discovery of TDP-43 has established the connection of both diseases on a neuropathological level.90 Up to 15% of ALS patients fulfill the FTD criteria,91 and a further 30% show mild cognitive or behavioral symptoms which do not fulfill the FTD criteria.91 Most commonly, executive abnormalities like deficits in verbal fluency92 are present. Furthermore, impairment in emotional and social cognition has been described.93 Cognitive deficits develop early in the disease process but progress slower than motor symptoms.94 Mild behavioral abnormalities most frequently present as apathy or reduced empathy95
In order to detect cognitive and behavioral deficits, many different screening batteries have been developed. Recently, the Edinburgh Cognitive and Behavioural Amyotrophic Lateral Sclerosis Screen has been established as a fast and reliable screening tool.96 Patients with cognitive deficits show frontotemporal white matter changes in cranial magnetic resonance imaging (MRI),97 and ALS patients frequently show executive oculomotor deficits which refer to a frontal and prefrontal pathology.98
Cognitive and behavioral symptoms commonly interfere with symptomatic treatment and worsen prognosis as highlighted in the respective sections. Furthermore, neurocognitive deficits may also impair decision making and managing of daily life. However, it has been shown that mild cognitive impairment does not affect decisions regarding acceptance or decline of PEG, NIV, and IV.45
Although symptomatic treatment options are not available for cognitive and behavioral abnormalities in ALS, it is important to detect and consider neuropsychological deficits throughout the disease process. Information about the disease in general and specific treatment options have to be adapted to the patient’s communication ability and mental capacity. Carers and healthcare professionals should be informed about the existence of cognitive and behavioral symptoms and their implications for ALS management and daily life.
Discussion
ALS therapy is a complex multidisciplinary challenge which is best managed by a team of specialized health care professionals. Nonpharmacological aspects including personal information, social and psychological support, aid advice, involvement of relatives and caregivers as well as guidance regarding end-of-life decisions and advance directives are of crucial importance in ALS and have been highlighted in the respective sections. Recent studies have confirmed the value of holistic, multidisciplinary treatment approaches, which have to be further developed, refined, and evaluated.
Since all phase III studies investigating potential disease-modifying drugs have been unsuccessful over the last few years, riluzole remains the only life-prolonging treatment to date and is the standard therapy for ALS in a dose of 2 × 50 mg per day. Edaravone slows down the disease in a well-defined subgroup of early ALS patients, but to date there is no oral administration form and it has not been approved in Europe yet. Antisense nucleotides offer a promising therapy option at least for the subgroup of patients with familial ALS, but the corresponding studies are only beginning to be initiated.
NIV has been established as a very important cornerstone in the treatment of patients with respiratory insufficiency, improving survival as well as quality of life. However, many questions regarding initiation of NIV, optimal ventilation parameters, and strategies to overcome individual ventilation barriers remain unresolved and urgently require further research. Diaphragm pacing has been proven to be futile (and probably even harmful) in ALS. This re-emphasizes the need for randomized controlled studies of symptomatic treatment measures.
Regarding nutrition, the importance of maintaining body weight is now widely accepted, and placebo-controlled multicenter studies are currently underway to prove the benefit of a high-caloric diet. However, further research is needed to answer the question whether or not high-lipid nutrition is beneficial. PEG, RIG, and PIG all have proven to be equally reliable and safe methods of enteral tube feeding in ALS. Further research is needed to determine optimal periprocedural treatment and the impact on survival and quality of life.
There are many different symptomatic treatment options for common symptoms like sialorrhea, spasticity, cramps, and pain, but evidence levels are generally low and rely heavily on case series and expert opinions in most instances. Therefore, therapies for these common symptoms vary greatly even between specialized centers and largely depend on local experience and expertise. However, we tried to extract the most accepted and reliable treatment options from the literature and available guidelines. These are summarized in Table 1. The table also contains treatment options of additional symptoms like pseudobulbar emotional lability or depression which have not been discussed in detail in the text. It combines recommendations from guidelines and our own experience.
Table 1.
Symptomatic therapy option in ALS.
| Symptom | Therapy options |
|---|---|
| Sialorrhea | transdermal scopolamine 1.5 mg, change every third day amitriptyline, 25–75 mg/d atropine drops 1% sublingually, 3–4 times/d botulinum toxin B injections in parotid or submandibular gland |
| Tenacious bronchial secretions | humidification of air / increased intake of fluids manual-assisted cough mechanical insufflator-exsufflator devices / home suction devices N-acetylcysteine 200 mg, 1–3 times/d nebulizers with saline / bronchodilatators / mucolytics |
| Spasticity | physiotherapy baclofen, starting with 5 mg/d, increase slowly tizanidine, starting with 2 mg/d, increase slowly patients with extrapyramidal involvement: L-Dopa, 100–200 mg/d |
| Muscle cramps | mexiletine, 300 mg/d quinine, 200–400 mg/d physiotherapy |
| Pseudobulbar emotional lability | SSRI (e.g. citalopram) 20–40 mg/d amitriptyline, 25–75 mg/d |
| Depression | SSRI (e.g. citalopram) 20–40 mg/d amitriptyline, 25–75 mg/d mirtazapine, 15–30 mg/d |
| Anxiety | Oral or sublingual lorazepam, 0.5–1 mg, 1–3 times/d (Note: respiratory depression) |
| Insomnia | amitriptyline, 25–75 mg/d zopiclone, 3.75–7.5 mg zolpidem, 5–10 mg (Note: respiratory depression) |
| Pain | NSAID and opioids according to WHO analgesic ladder (Note: respiratory depression) |
ALS, amyotrophic lateral sclerosis; NSAID, nonsteroidal anti-inflammatory drug; SSRI, selective serotonin reuptake inhibitor; WHO, World Health Organization
Acknowledgments
We thank Patrick Weydt for his review and helpful comments.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Johannes Dorst, Universitätsklinik Ulm, RKU, Oberer Eselsberg 45, D-89081 Ulm, Germany.
Albert C. Ludolph, Universitätsklinik Ulm, Ulm, Germany
Annemarie Huebers, Universitätsklinik Ulm, Ulm, Germany.
References
- 1. Forsgren L, Almay BG, Holmgren G, et al. Epidemiology of motor neuron disease in northern Sweden. Acta Neurol Scand 1983; 68: 20–29. [DOI] [PubMed] [Google Scholar]
- 2. Haverkamp LJ, Appel V, Appel SH. Natural history of amyotrophic lateral sclerosis in a database population. Validation of a scoring system and a model for survival prediction. Brain 1995; 118: 707–719. [DOI] [PubMed] [Google Scholar]
- 3. Feneberg E, Hübers A, Weishaupt JH, et al. Genetik und Neurochemische Biomarker bei Amyotropher Lateralsklerose und Frontotemporaler Lobärdegeneration. Akt Neurol 2014; 41: 239–247. [Google Scholar]
- 4. Braak H, Brettschneider J, Ludolph AC, et al. Amyotrophic lateral sclerosis–a model of corticofugal axonal spread. Nat Rev Neurol 2013; 9: 708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ludolph A, Drory V, Hardiman O, et al. A revision of the El Escorial criteria - 2015. Amyotroph Lateral Scler Frontotemporal Degener 2015: 1–2. [DOI] [PubMed] [Google Scholar]
- 6. Miller RG, Jackson CE, Kasarskis EJ, et al. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2009; 73: 1218–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chio A, Bottacchi E, Buffa C, et al. Positive effects of tertiary centres for amyotrophic lateral sclerosis on outcome and use of hospital facilities. J Neurol Neurosurg Psychiatry 2006; 77: 948–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cudkowicz ME, van den Berg LH, Shefner JM, et al. Dexpramipexole versus placebo for patients with amyotrophic lateral sclerosis (EMPOWER): a randomised, double-blind, phase 3 trial. Lancet Neurol 2013; 12: 1059–1067. [DOI] [PubMed] [Google Scholar]
- 9. Dupuis L, Dengler R, Heneka MT, et al. A randomized, double blind, placebo-controlled trial of pioglitazone in combination with riluzole in amyotrophic lateral sclerosis. PloS One 2012; 7: e37885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morrison KE, Dhariwal S, Hornabrook R, et al. Lithium in patients with amyotrophic lateral sclerosis (LiCALS): a phase 3 multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol 2013; 12: 339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zoccolella S, Beghi E, Palagano G, et al. Riluzole and amyotrophic lateral sclerosis survival: a population-based study in southern Italy. Eur J Neurol 2007; 14: 262–268. [DOI] [PubMed] [Google Scholar]
- 12. Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med 1994; 330: 585–591. [DOI] [PubMed] [Google Scholar]
- 13. Lacomblez L, Bensimon G, Leigh PN, et al. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet 1996; 347: 1425–1431. [DOI] [PubMed] [Google Scholar]
- 14. Miller RG, Mitchell JD, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst Rev 2012; 3: CD001447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dyer AM, Smith A. Riluzole 5 mg/mL oral suspension: for optimized drug delivery in amyotrophic lateral sclerosis. Drug Des Devel Ther 2016; 11: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Edavarone StudyGroup. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2017; 16: 505–512. [DOI] [PubMed] [Google Scholar]
- 17. Jimenez-Pacheco A, Franco JM, Lopez S, et al. Epigenetic mechanisms of gene regulation in amyotrophic lateral sclerosis. Adv Exp Med Biol 2017; 978: 255–275. [DOI] [PubMed] [Google Scholar]
- 18. Bossy-Wetzel E, Schwarzenbacher R, Lipton SA. Molecular pathways to neurodegeneration. Nat Med 2004; 10(Suppl.): S2–S9. [DOI] [PubMed] [Google Scholar]
- 19. Hubers A, Weishaupt JH, Ludolph AC. Genetik der amyotrophen Lateralsklerose [Genetics of amyotrophic lateral sclerosis]. Der Nervenarzt 2013; 84: 1213–1219. [DOI] [PubMed] [Google Scholar]
- 20. Blokhuis AM, Groen EJ, Koppers M, et al. Protein aggregation in amyotrophic lateral sclerosis. Acta Neuropathol 2013; 125: 777–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smith RA, Miller TM, Yamanaka K, et al. Antisense oligonucleotide therapy for neurodegenerative disease. J Clin Invest 2006; 116: 2290–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Crooke ST. Progress in antisense technology. Ann Rev Med 2004; 55: 61–95. [DOI] [PubMed] [Google Scholar]
- 23. Evers MM, Toonen LJ, van Roon-Mom WM. Antisense oligonucleotides in therapy for neurodegenerative disorders. Adv Drug Deliv Rev 2015; 87: 90–103. [DOI] [PubMed] [Google Scholar]
- 24. Winer L, Srinivasan D, Chun S, et al. SOD1 in cerebral spinal fluid as a pharmacodynamic marker for antisense oligonucleotide therapy. JAMA Neurol 2013; 70: 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miller TM, Pestronk A, David W, et al. An antisense oligonucleotide against SOD1 delivered intrathecally for patients with SOD1 familial amyotrophic lateral sclerosis: a phase 1, randomised, first-in-man study. Lancet Neurol 2013; 12: 435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Zundert B, Brown RH., Jr. Silencing strategies for therapy of SOD1-mediated ALS. Neurosci Lett 2017; 636: 32–39. [DOI] [PubMed] [Google Scholar]
- 27. Chiriboga CA, Swoboda KJ, Darras BT, et al. Results from a phase 1 study of nusinersen (ISIS-SMN(Rx)) in children with spinal muscular atrophy. Neurology 2016; 86: 890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Desport JC, Preux PM, Truong TC, et al. Nutritional status is a prognostic factor for survival in ALS patients. Neurology 1999; 53: 1059–1063. [DOI] [PubMed] [Google Scholar]
- 29. Marin B, Desport JC, Kajeu P, et al. Alteration of nutritional status at diagnosis is a prognostic factor for survival of amyotrophic lateral sclerosis patients. J Neurol Neurosurg Psychiatry 2011; 82: 628–634. [DOI] [PubMed] [Google Scholar]
- 30. Desport JC, Preux PM, Magy L, et al. Factors correlated with hypermetabolism in patients with amyotrophic lateral sclerosis. Am J Clin Nutr 2001; 74: 328–334. [DOI] [PubMed] [Google Scholar]
- 31. Vercruysse P, Sinniger J, El Oussini H, et al. Alterations in the hypothalamic melanocortin pathway in amyotrophic lateral sclerosis. Brain 2016; 139: 1106–1122. [DOI] [PubMed] [Google Scholar]
- 32. Dupuis L, Corcia P, Fergani A, et al. Dyslipidemia is a protective factor in amyotrophic lateral sclerosis. Neurology 2008; 70: 1004–1009. [DOI] [PubMed] [Google Scholar]
- 33. Dorst J, Kuhnlein P, Hendrich C, et al. Patients with elevated triglyceride and cholesterol serum levels have a prolonged survival in amyotrophic lateral sclerosis. J Neurol 2011; 258: 613–617. [DOI] [PubMed] [Google Scholar]
- 34. Chio A, Calvo A, Ilardi A, et al. Lower serum lipid levels are related to respiratory impairment in patients with ALS. Neurology 2009; 73: 1681–1685. [DOI] [PubMed] [Google Scholar]
- 35. Paganoni S, Deng J, Jaffa M, et al. Body mass index, not dyslipidemia, is an independent predictor of survival in amyotrophic lateral sclerosis. Muscle Nerve 2011; 44: 20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zinman L, Sadeghi R, Gawel M, et al. Are statin medications safe in patients with ALS? Amyotroph Lateral Scler 2008; 9: 223–228. [DOI] [PubMed] [Google Scholar]
- 37. Dupuis L, Oudart H, Rene F, et al. Evidence for defective energy homeostasis in amyotrophic lateral sclerosis: benefit of a high-energy diet in a transgenic mouse model. Proc Natl Acad Sci USA 2004; 101: 11159–11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dorst J, Cypionka J, Ludolph AC. High-caloric food supplements in the treatment of amyotrophic lateral sclerosis: a prospective interventional study. Amyotroph Lateral Scler Frontotemporal Degener 2013; 14: 533–536. [DOI] [PubMed] [Google Scholar]
- 39. Lopez Gomez JJ, Ballesteros Pomar MD, Vazquez Sanchez F, et al. Efecto del soporte nutricional sobre la supervivencia en pacientes con esclerosis lateral amiotrofica [Effect of nutritional support on survival in patients with amyotrophic lateral sclerosis]. Nutr Hosp 2011; 26: 515–521. [DOI] [PubMed] [Google Scholar]
- 40. Andersen PM, Abrahams S, Borasio GD, et al. EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS)–revised report of an EFNS task force. Eur J Neurol 2012; 19: 360–375. [DOI] [PubMed] [Google Scholar]
- 41. Stavroulakis T, Walsh T, Shaw PJ, et al. Gastrostomy use in motor neurone disease (MND): a review, meta-analysis and survey of current practice. Amyotroph Lateral Scler Frontotemporal Degener 2013; 14: 96–104. [DOI] [PubMed] [Google Scholar]
- 42. ProGas. Gastrostomy in patients with amyotrophic lateral sclerosis (ProGas): a prospective cohort study. Lancet Neurol 2015; 14: 702–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dorst J, Dupuis L, Petri S, et al. Percutaneous endoscopic gastrostomy in amyotrophic lateral sclerosis: a prospective observational study. J Neurol 2015; 262: 849–858. [DOI] [PubMed] [Google Scholar]
- 44. Czell D, Bauer M, Binek J, et al. Outcomes of percutaneous endoscopic gastrostomy tube insertion in respiratory impaired amyotrophic lateral sclerosis patients under noninvasive ventilation. Respir Care 2013; 58: 838–844. [DOI] [PubMed] [Google Scholar]
- 45. Bohm S, Aho-Ozhan HE, Keller J, et al. Medical decisions are independent of cognitive impairment in amyotrophic lateral sclerosis. Neurology 2016; 87: 1737–1738. [DOI] [PubMed] [Google Scholar]
- 46. Wills AM, Hubbard J, Macklin EA, et al. Hypercaloric enteral nutrition in patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet 2014; 383: 2065–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lipp A, Lusardi G. Systemic antimicrobial prophylaxis for percutaneous endoscopic gastrostomy. Cochrane Database Syst Rev 2013; 11: CD005571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bourke SC, Tomlinson M, Williams TL, et al. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. Lancet Neurol 2006; 5: 140–147. [DOI] [PubMed] [Google Scholar]
- 49. Berlowitz DJ, Howard ME, Fiore JF, Jr, et al. Identifying who will benefit from non-invasive ventilation in amyotrophic lateral sclerosis/motor neurone disease in a clinical cohort. J Neurol Neurosurg Psychiatry 2016; 87: 280–286. [DOI] [PubMed] [Google Scholar]
- 50. Boentert M, Brenscheidt I, Glatz C, et al. Effects of non-invasive ventilation on objective sleep and nocturnal respiration in patients with amyotrophic lateral sclerosis. J Neurol 2015; 262: 2073–2082. [DOI] [PubMed] [Google Scholar]
- 51. Butz M, Wollinsky KH, Wiedemuth-Catrinescu U, et al. Longitudinal effects of noninvasive positive-pressure ventilation in patients with amyotrophic lateral sclerosis. Am J Phys Med Rehabil 2003; 82: 597–604. [DOI] [PubMed] [Google Scholar]
- 52. Mustfa N, Walsh E, Bryant V, et al. The effect of noninvasive ventilation on ALS patients and their caregivers. Neurology 2006; 66: 1211–1217. [DOI] [PubMed] [Google Scholar]
- 53. Chio A, Ilardi A, Cammarosano S, et al. Neurobehavioral dysfunction in ALS has a negative effect on outcome and use of PEG and NIV. Neurology 2012; 78: 1085–1089. [DOI] [PubMed] [Google Scholar]
- 54. Volanti P, Cibella F, Sarva M, et al. Predictors of non-invasive ventilation tolerance in amyotrophic lateral sclerosis. J Neurol Sci 2011; 303: 114–118. [DOI] [PubMed] [Google Scholar]
- 55. Peysson S, Vandenberghe N, Philit F, et al. Factors predicting survival following noninvasive ventilation in amyotrophic lateral sclerosis. Eur Neurol 2008; 59: 164–171. [DOI] [PubMed] [Google Scholar]
- 56. Winck JC, Goncalves MR, Lourenco C, et al. Effects of mechanical insufflation-exsufflation on respiratory parameters for patients with chronic airway secretion encumbrance. Chest 2004; 126: 774–780. [DOI] [PubMed] [Google Scholar]
- 57. Baxter SK, Baird WO, Thompson S, et al. The initiation of non-invasive ventilation for patients with motor neuron disease: patient and carer perceptions of obstacles and outcomes. Amyotroph Lateral Scler Frontotemporal Degener 2013; 14: 105–110. [DOI] [PubMed] [Google Scholar]
- 58. Sancho J, Servera E, Morelot-Panzini C, et al. Non-invasive ventilation effectiveness and the effect of ventilatory mode on survival in ALS patients. Amyotroph Lateral Scler Frontotemporal Degener 2014; 15: 55–61. [DOI] [PubMed] [Google Scholar]
- 59. Spataro R, Bono V, Marchese S, et al. Tracheostomy mechanical ventilation in patients with amyotrophic lateral sclerosis: clinical features and survival analysis. J Neuroll Sci 2012; 323: 66–70. [DOI] [PubMed] [Google Scholar]
- 60. Neudert C, Oliver D, Wasner M, et al. The course of the terminal phase in patients with amyotrophic lateral sclerosis. J Neurol 2001; 248: 612–616. [DOI] [PubMed] [Google Scholar]
- 61. Gonzalez-Bermejo J, Morelot-Panzini C, Salachas F, et al. Diaphragm pacing improves sleep in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2012; 13: 44–54. [DOI] [PubMed] [Google Scholar]
- 62. DiPALS. Safety and efficacy of diaphragm pacing in patients with respiratory insufficiency due to amyotrophic lateral sclerosis (DiPALS): a multicentre, open-label, randomised controlled trial. Lancet Neurol 2015; 14: 883–892. [DOI] [PubMed] [Google Scholar]
- 63. Gonzalez-Bermejo J, Morelot-Panzini C, Tanguy ML, et al. Early diaphragm pacing in patients with amyotrophic lateral sclerosis (RespiStimALS): a randomised controlled triple-blind trial. Lancet Neurol 2016; 15: 1217–1227. [DOI] [PubMed] [Google Scholar]
- 64. Drory VE, Goltsman E, Reznik JG, et al. The value of muscle exercise in patients with amyotrophic lateral sclerosis. J Neurol Sci 2001; 191: 133–137. [DOI] [PubMed] [Google Scholar]
- 65. Bello-Haas VD, Florence JM, Kloos AD, et al. A randomized controlled trial of resistance exercise in individuals with ALS. Neurology 2007; 68: 2003–2007. [DOI] [PubMed] [Google Scholar]
- 66. Lunetta C, Lizio A, Sansone VA, et al. Strictly monitored exercise programs reduce motor deterioration in ALS: preliminary results of a randomized controlled trial. J Neurol 2016; 263: 52–60. [DOI] [PubMed] [Google Scholar]
- 67. Dal Bello-Haas V, Florence JM. Therapeutic exercise for people with amyotrophic lateral sclerosis or motor neuron disease. Cochrane Database Syst Rev 2013; 5: CD005229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mahoney DJ, Rodriguez C, Devries M, et al. Effects of high-intensity endurance exercise training in the G93A mouse model of amyotrophic lateral sclerosis. Muscle Nerve 2004; 29: 656–662. [DOI] [PubMed] [Google Scholar]
- 69. Suryadevara U, Bruijnzeel DM, Nuthi M, et al. Pros and cons of medical cannabis use by people with chronic brain disorders. Curr Neuropharmacol 2017; 15: 800–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pradat PF, Bruneteau G, Munerati E, et al. Extrapyramidal stiffness in patients with amyotrophic lateral sclerosis. Mov Disord 2009; 24: 2143–2148. [DOI] [PubMed] [Google Scholar]
- 71. Vazquez-Costa JF, Manez I, Alabajos A, et al. Safety and efficacy of botulinum toxin A for the treatment of spasticity in amyotrophic lateral sclerosis: results of a pilot study. J Neurol 2016; 263: 1954–1960. [DOI] [PubMed] [Google Scholar]
- 72. Baldinger R, Katzberg HD, Weber M. Treatment for cramps in amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev 2012: CD004157. [DOI] [PubMed] [Google Scholar]
- 73. Weiss MD, Macklin EA, Simmons Z, et al. A randomized trial of mexiletine in ALS: safety and effects on muscle cramps and progression. Neurology 2016; 86: 1474–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ribeiro S. Iyengar yoga therapy as an intervention for cramp management in individuals with amyotrophic lateral sclerosis: three case reports. J Altern Complement Med 2014; 20: 322–326. [DOI] [PubMed] [Google Scholar]
- 75. Jackson CE, Gronseth G, Rosenfeld J, et al. Randomized double-blind study of botulinum toxin type B for sialorrhea in ALS patients. Muscle Nerve 2009; 39: 137–143. [DOI] [PubMed] [Google Scholar]
- 76. Guidubaldi A, Fasano A, Ialongo T, et al. Botulinum toxin A versus B in sialorrhea: a prospective, randomized, double-blind, crossover pilot study in patients with amyotrophic lateral sclerosis or Parkinson’s disease. Mov Disord 2011; 26: 313–319. [DOI] [PubMed] [Google Scholar]
- 77. Winterholler MG, Erbguth FJ, Wolf S, et al. Botulinum toxin for the treatment of sialorrhoea in ALS: serious side effects of a transductal approach. J Neurol Neurosurg Psychiatry 2001; 70: 417–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Guy N, Bourry N, Dallel R, et al. Comparison of radiotherapy types in the treatment of sialorrhea in amyotrophic lateral sclerosis. J Palliat Med 2011; 14: 391–395. [DOI] [PubMed] [Google Scholar]
- 79. Kasarskis EJ, Hodskins J, St Clair WH. Unilateral parotid electron beam radiotherapy as palliative treatment for sialorrhea in amyotrophic lateral sclerosis. J Neurol Sci 2011; 308: 155–157. [DOI] [PubMed] [Google Scholar]
- 80. Bourry N, Guy N, Achard JL, et al. Salivary glands radiotherapy to reduce sialorrhea in amyotrophic lateral sclerosis: dose and energy. Cancer Radiother 2013; 17: 191–195. [DOI] [PubMed] [Google Scholar]
- 81. Assouline A, Levy A, Abdelnour-Mallet M, et al. Radiation therapy for hypersalivation: a prospective study in 50 amyotrophic lateral sclerosis patients. Int J Radiat Oncol Biol Phys 2014; 88: 589–595. [DOI] [PubMed] [Google Scholar]
- 82. Hawkey NM, Zaorsky NG, Galloway TJ. The role of radiation therapy in the management of sialorrhea: a systematic review. Laryngoscope 2016; 126: 80–85. [DOI] [PubMed] [Google Scholar]
- 83. Hobson EV, McGeachan A, Al-Chalabi A, et al. Management of sialorrhoea in motor neuron disease: a survey of current UK practice. Amyotroph Lateral Scler Frontotemporal Degener 2013; 14: 521–527. [DOI] [PubMed] [Google Scholar]
- 84. Newall AR, Orser R, Hunt M. The control of oral secretions in bulbar ALS/MND. J Neurol Sci 1996; 139: 43–44. [DOI] [PubMed] [Google Scholar]
- 85. Sancho J, Servera E, Diaz J, et al. Efficacy of mechanical insufflation-exsufflation in medically stable patients with amyotrophic lateral sclerosis. Chest 2004; 125: 1400–1405. [DOI] [PubMed] [Google Scholar]
- 86. Ganzini L, Johnston WS, Hoffman WF. Correlates of suffering in amyotrophic lateral sclerosis. Neurology 1999; 52: 1434–1440. [DOI] [PubMed] [Google Scholar]
- 87. Brettschneider J, Kurent J, Ludolph A. Drug therapy for pain in amyotrophic lateral sclerosis or motor neuron disease. Cochrane Database Syst Rev 2013: CD005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Cancer pain relief and palliative care. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser 1990; 804: 1–75. [PubMed] [Google Scholar]
- 89. Oliver D. Opioid medication in the palliative care of motor neurone disease. Palliat Med 1998; 12: 113–115. [DOI] [PubMed] [Google Scholar]
- 90. Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006; 314: 130–133. [DOI] [PubMed] [Google Scholar]
- 91. Ringholz GM, Appel SH, Bradshaw M, et al. Prevalence and patterns of cognitive impairment in sporadic ALS. Neurology 2005; 65: 586–590. [DOI] [PubMed] [Google Scholar]
- 92. Abrahams S, Leigh PN, Goldstein LH. Cognitive change in ALS: a prospective study. Neurology 2005; 64: 1222–1226. [DOI] [PubMed] [Google Scholar]
- 93. Gibbons ZC, Richardson A, Neary D, et al. Behaviour in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2008; 9: 67–74. [DOI] [PubMed] [Google Scholar]
- 94. Schreiber H, Gaigalat T, Wiedemuth-Catrinescu U, et al. Cognitive function in bulbar- and spinal-onset amyotrophic lateral sclerosis. A longitudinal study in 52 patients. J Neurol 2005; 252: 772–781. [DOI] [PubMed] [Google Scholar]
- 95. Bock M, Duong YN, Kim A, et al. Cognitive-behavioral changes in amyotrophic lateral sclerosis: screening prevalence and impact on patients and caregivers. Amyotroph Lateral Scler Frontotemporal Degener 2016; 17: 366–373. [DOI] [PubMed] [Google Scholar]
- 96. Lule D, Burkhardt C, Abdulla S, et al. The Edinburgh cognitive and behavioural amyotrophic lateral sclerosis screen: a cross-sectional comparison of established screening tools in a German-Swiss population. Amyotroph Lateral Scler Frontotemporal Degener 2015; 16: 16–23. [DOI] [PubMed] [Google Scholar]
- 97. Abrahams S, Goldstein LH, Suckling J, et al. Frontotemporal white matter changes in amyotrophic lateral sclerosis. J Neurol 2005; 252: 321–331. [DOI] [PubMed] [Google Scholar]
- 98. Gorges M, Muller HP, Lule D, et al. Eye movement deficits are consistent with a staging model of pTDP-43 pathology in amyotrophic lateral sclerosis. PloS One 2015; 10: e0142546. [DOI] [PMC free article] [PubMed] [Google Scholar]