Fig. 5.
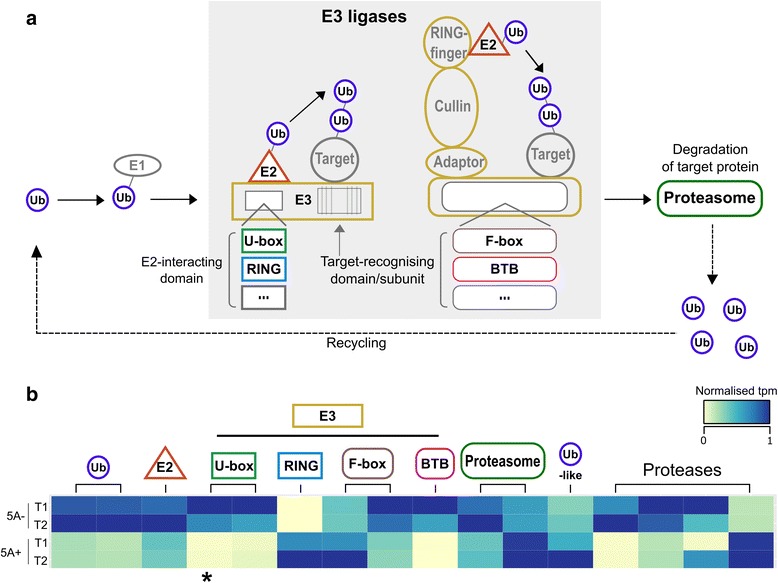
Differential regulation of the ubiquitin pathway in 5A NILs. a Differentially expressed (DE) transcripts with functional annotations related to ubiquitin-mediated protein turnover were enriched relative to the whole genome (a). This pathway acts to add multiple copies of the protein Ubiquitin (Ub) to a substrate protein through the sequential action of a cascade of three enzymes: E1 (Ub-activating enzymes), E2 (Ub-conjugating enzymes) and E3 (Ub ligases). The tagged substrate is then targeted for degradation by the 26S proteasome and the Ub proteins are recycled. The E3 ligases are the most diverse of the three enzymes and both single subunit proteins and multi-subunit complexes exist. A subset of these classes is shown in the grey box in (a), selected based on the annotations of DE transcripts. Single subunit E3 ligases have an E2-interacting domain (e.g. U-box, RING, etc. (…)) and a substrate-recognising domain. Multi-subunit complexes also have E2-interacting complexes and substrate-recognising subunits (e.g. F-box, BTB, etc. (…)). In the context of organ size control, some proteases have been identified as downstream targets of this pathway (e.g. DA1, UBP15 [50, 51]). b Heatmap of normalised tpm of DE transcripts associated with ubiquitin, the proteasome and proteases. * indicates the transcript located in the fine-mapped region of the 5A grain length QTL (Fig. 4b; RING/U-box superfamily protein)
