Abstract
Since its discovery, the human epidermal growth factor 2 (HER2) has been extensively studied. Presently, there are 2 standard diagnostic techniques to assess HER2 status in biopsies: immunohistochemistry and fluorescence in situ hybridization. While these techniques have played an important role in the treatment of patients with HER2-positive cancer, they both require invasive biopsies for analysis. Moreover, the expression of HER2 is heterogeneous in breast cancer and can change over the course of the disease. Thus, the degree of HER2 expression in the small sample size of biopsied tumors at the time of analysis may not represent the overall status of HER2 expression in the whole tumor and in between tumor foci in the metastatic setting as the disease progresses. Unlike biopsy, molecular imaging using probes against HER2 allows for a noninvasive, whole-body assessment of HER2 status in real time. This technique could potentially select patients who may benefit from HER2-directed therapy and offer alternative treatments to those who may not benefit. Several antibodies and small molecules against HER2 have been labeled with different radioisotopes for nuclear imaging and/or therapy. This review presents the most recent advances in HER2 targeting in nuclear medicine focusing on preclinical and clinical studies.
Keywords: breast cancer, HER2-positive cancer, HER2 imaging, human epidermal growth factor 2, molecular imaging, PET/CT, SPECT/CT, receptor radionuclide therapy
Introduction
The human epidermal growth factor 2 (HER2) has been extensively studied since its discovery in 1987 by Dr Slamon and colleagues,1 mainly as its overexpression in tumors has been associated with more aggressive tumor types. Human epidermal growth factor 2 is also known as erbB-2 and Neu; it is a member of the ErbBs or type I receptor tyrosine kinase family, which also includes the epidermal growth factor receptor 1 (EGFR or HER1), erbB-3 (HER3), and erbB-4 (HER4).2,3 The HER family includes transmembrane proteins that activate intracellular signaling pathways in response to extracellular signals. Their structure consists of an extracellular ligand-binding domain, a transmembrane domain, and an intracellular tyrosine kinase domain. These proteins are expressed in a variety of tissues of epithelial, mesenchymal, and neuronal origin, where they are involved in cell development, proliferation, and differentiation.2,4,5 Some cancer cells exhibit amplification of the HER2 gene, which often leads to the overexpression of the HER2 protein on the cell surface. This overexpression occurs in several cancers, including bladder, lung, gastric, ovarian, prostate, and breast cancer (BCa).6 Tumor cells that overexpress HER2 frequently have a high rate of proliferation and are associated with more aggressive disease, poor prognosis, and shorter overall survival.7,8 The development of HER2-targeted treatments using monoclonal antibodies (mAbs) has significantly improved patient survival, particularly in up to 20% of patients with BCa.9,10 Patients with the triple negative subtype of BCa, whose tumors do not express estrogen receptor, progesterone receptor, and HER2,11 cannot take advantage of HER2-directed therapies. Molecular imaging using anti-HER2 agents cannot diagnose the triple negative subtypes; however, it can exclude those who will not benefit from anti-HER2 therapy such that patients can have alternative treatments. As new targeted agents are currently being developed for the HER2-negative subtypes of BCa, molecular imaging approaches of emerging biomarkers have the potential to predict response to these investigational treatments. The lessons we learn from developing imaging agents for HER2 can be applied to future imaging agents for the HER2-negative subtypes of BCa.
Trastuzumab (Herceptin; Genentech, South San Francisco, CA) was the first humanized mAb against HER2 to be approved by the Food and Drug Administration (FDA) for HER2-positive BCa. Trastuzumab binds to domain IV of the extracellular domain of the HER2 epitope which ultimately suppresses cancer cell proliferation, growth, and survival through the following mechanism of actions: (1) downregulating total levels of HER2; (2) blocking cleavage of the extracellular domain of HER2; (3) inhibiting HER2 homodimerization, which inhibits the PI3K intracellular signaling pathway; (4) reducing angiogenesis; and finally (5) inducing antibody-dependent cellular cytotoxicity or lysing the antibody-bound cells via recruitment of immune cells.12,13
Despite the success of trastuzumab, HER2-targeted therapy remains a challenge. A significant number of patients are primarily resistant to this drug (intrinsic resistance), and prolonged treatment often ends with the majority of patients who initially had a clinical benefit becoming resistant (acquired resistance). The mechanisms of intrinsic resistance to trastuzumab are most often associated with an inactive target, whereas acquired resistance mostly occurs due to modifications in the target signaling level or the loss of HER2 expression over the course of the disease.10 Another important limitation of trastuzumab is that some patients can have cardiotoxicity, as HER2 is expressed in the heart.14 Studies in tumor-bearing mice cannot determine cardiotoxicity because trastuzumab is not cross-reactive with murine HER2.
In order to overcome this resistance in advanced disease, other drugs can be combined with trastuzumab. Pertuzumab, trastuzumab emtansine (T-DM1), and lapatinib are approved for inhibiting HER2 activity in the treatment of HER2-positive metastatic BCa. Pertuzumab (Perjeta; Genentech, South San Francisco, CA) is a humanized mAb that binds to domain II of the extracellular domain of the HER2 epitope and functions by inhibiting HER2 dimerization with other growth factor receptors, particularly the HER2-HER3 dimerization.15 Preclinical experiments showed that the combination of trastuzumab and pertuzumab enhanced the antitumor effect compared to trastuzumab or pertuzumab alone due to complementary mechanisms of action that promote tumor regression more effectively.15 This combination has also been tested in patients with metastatic BCa in a recent study with the CLEOPATRA trial.16 The CLEOPATRA trial showed that patients who received trastuzumab, pertuzumab, and docetaxel (or paclitaxel) had a median overall survival of 56.5 months compared to 15 months for those who received trastuzumab alone. Since this trial, the combination of trastuzumab, pertuzumab, and docetaxel is used as first-line therapy for metastatic HER2-positive BCa.16
Another approach to overcoming resistance is the use of antibody drug conjugates, whereby antigen binding on the cell surface mediates the internalization of the antibody and subsequent delivery of the toxic payload to increase the selectivity and potency of this drug. The FDA has approved T-DM1 as second-line therapy for patients with HER2-positive metastatic BCa who previously received trastuzumab. DM1 (a derivative of maytansine) is a potent inhibitor of microtubule polymerization and is linked to trastuzumab to form the T-DM1 antibody–drug conjugate. T-DM1 has been well tolerated by patients. The only grade ≥3 adverse events are reversible thrombocytopenia (decrease in platelet count) and elevations in hepatic transaminase, which are present in ≥5% of patients.17 Currently, there are new drugs under evaluation in clinical trials which might give new options to the treatment of advanced HER2-positive BCa such as HER2 vaccines, new antibodies (ertumaxomab and margetuximab), and defucosylated trastuzumab.3
The availability of several drugs targeting HER2 on the extracellular and/or intracellular domain provides several options for treatment, which places nuclear medicine in a unique position to help guide decisions for treatment. Targeting HER2 for imaging and/or therapy in nuclear medicine has generated a wide interest, resulting in many recent studies with a focus on developing new radiopharmaceuticals. This review highlights recent advances in HER2-targeted imaging and therapy from both preclinical and clinical oncological studies published since 2015.
Radiopharmaceuticals Targeting HER2 for Diagnostic Use
It is evident that an accurate characterization of HER2 expression is the key for the success of HER2-targeted therapy. At present, there are 2 types of tests to determine the HER2 status in BCa: (1) immunohistochemistry (IHC), which detects the HER2 protein and (2) fluorescence in situ hybridization (FISH), which detects the copy number of the HER2 gene.18 However, it has been reported that up to 20% of results using these methods may be inaccurate.18 Both methods are performed on biopsied tissues. Mathenge et al demonstrated that the use of core needle biopsy before surgical excision of BCa tumors can significantly increase lung metastasis in mice.19 Therefore, biopsy may unintentionally promote metastasis by dissemination of cancer cells from the primary lesion to distant organs.19 Many protocols encourage the use of repeated biopsies during the course of treatment, since HER2 expression can change over the course of the disease.
In addition, intratumoral heterogeneity and small sample size of biopsied tumors may not represent the status of HER2 expression in the whole tumor or between tumor foci in the metastatic setting.6,20 Thus, a more accurate method for assessment of the HER2 status is needed.
In this scenario, molecular imaging using specific radiopharmaceuticals to target HER2 exhibits an immense advantage. This new technique may provide a complementary and noninvasive option to identify patients who may be responsive and those who may not respond to HER2-targeted therapy. Since molecular imaging is a noninvasive procedure, it may have an advantage over biopsy-based approaches and has the potential to help guide physicians to tailor the treatment for each patient. Furthermore, it may be possible to monitor the response to the HER2-targeted therapy and identify those patients who become resistant.
Anti-HER2 probes have the ability to bind to the HER2 protein regardless of its gene amplification. Imaging HER2 using these agents is an advantage over FISH, as gene amplification does not always lead to the overexpression of the HER2 protein.21,22 Also, some somatic mutations can lead to a negative result in gene amplification, while the tumor cells continue to express the HER2 protein.21
Several studies have been published using radiolabeled intact mAbs, antibody fragments (for instance, Fab), nanobodies, and affibodies for the development of new imaging agents for HER2. Low-molecular-weight constructs in these studies have been developed to reduce the blood residency time to allow for imaging at earlier time points (hours) than that achieved by intact mAb (days). Tables 1 and 2 summarize the results of the main studies performed in preclinical and clinical stages.
Table 1.
Preclinical Studies Performed With Probes Against HER2 Receptor.
| Probe, Dose, and Modality | Main Findings | Study |
|---|---|---|
| 64Cu-NOTA-pertuzumab, F(ab′)2; 1-3 MBq; PET | High accumulation in the kidneys; predicted total body dose in humans was 0.015 mSv/MBq | Lam et al23 |
| 89Zr-HOPO-trastuzumab; 0.5 MBq; PET | Good tumor uptake despite lower purity and stability | Tinianow et al (2016)24 |
| 64Cu-NOTA-Fab-PEG24-EGF; 15-25 MBq; PET | High accumulation in the liver and kidneys 48 hours PI; clear visualization of tumor xenografts expressing one or both receptors (PI HER2 and EGF) | Kwon et al25 |
| 177Lu-trastuzumab-AuNP; 3 MBq, intratumorally injection; therapy | In xenograft BCa tumors, the DNA damage caused by the gold nanoparticles modified with trastuzumab was at least 2.8-fold higher than the nanoparticle without trastuzumab | Cai et al26 |
| 111In-trastuzumab-AuNP; 10 MBq, intratumorally injection; therapy | 111In-trastuzumab-AuNP inhibited tumor growth in mice with SC HER2-positive BC xenografts; no toxicity was found in normal tissues | Cai et al27 |
| 177Lu-DOTA-Fab-PEG24-EGF and 111In-DOTA-Fab-PEG24-EGF; 11.1 MBq, intraperitoneal injection; therapy | 177Lu-DOTA-Fab-PEG24-EGF demonstrated stronger tumor growth inhibition than 111In-DOTA-Fab-PEG24-EGF even in tumors that are trastuzumab resistant | Razumienko et al28 |
| 99mTc-HYNIC-H6F; 37 MBq; SPECT | Peptide demonstrated excellent HER2 binding specificity both in vitro and in vivo. Uptake in tumor was not blocked by coinjection of excess of trastuzumab | Li et al29 |
| 99mTc-CGGG-LTVSPWY and 99mTc-CSSS-LTVSPWY; 7.4 MBq; SPECT | Both peptides showed specific binding; the CSSS ligand showed more favorable uptake in the tumor | Sabahnoo et al30 |
| 99mTc-trastuzumab-PCSN; 5.9 MBq; SPECT | Good uptake in tumor; poor radiochemical yield | Yamaguchi et al20 |
| 64Cu-NOTA-pertuzumab; 5-10 MBq; PET | Clear tumor visualization including tumors orthotropic in the peritoneal cavity | Jiang et al31 |
| 177Lu-pertuzumab; 5-7 MBq; therapy | Specific and high tumor uptake; elevated absorbed dose in tumors contributing to the inhibition of tumor progression | Persson et al32 |
| 89Zr-pertuzumab; 3.7 MBq; PET | Optimal image timing: 7 days PI; tumor uptake was increased in presence of unlabeled trastuzumab | Marquez et al33 |
| 90Y-CHX-A″-DTPA-trastuzumab and 90Y-octapa-trastuzumab; 3.7 MBq; therapy | Both chelators provided high radiochemical yields; high tumor uptake after 72 hours PI; significant decrease in tumor growth compared to controls after 36 days of therapy | Price et al34 |
| 131I-trastuzumab; 0.7-0.55 MBq; biodistribution evaluation | Good affinity for HER2-positive cells; immunoreactivity not compromised; significant tumor uptake after 24 hours PI; high uptake in the liver, lungs, and spleen | Kameswaran et al35 |
| 111In-trastuzumab-NLS-S and -L; 0.37 MBq; therapy; cytotoxicity | 111In-trastuzumab-NLS showed higher cytotoxicity compared to 111In-trastuzumab and cytotoxicity was enhanced in the presence of bortezomid | Li et al36 |
| 188Re-HYNIC-trastuzumab; 0.037-0.74 MBq; cell therapy | 188Re-HYNIC-trastuzumab enhanced the cytotoxicity to nearly 100-fold than trastuzumab alone; 188Re-HYNIC-trastuzumab prolongs the effects of apoptosis | Luo et al37 |
Abbreviations: EGF, epidermal growth factor; HER2, human epidermal growth factor 2; PET, positron emission tomography; PI, postinjection; SC, subcutaneous; SPECT, single-photon emission computed tomography.
Table 2.
Clinical Studies Performed With Probes Against HER2 Receptor.
| Probe, Dose, and Modality | Patient Population | Main Findings | Study |
|---|---|---|---|
| 89Zr-trastuzumab; 185 MBq; PET | Metastatic HER2-negative primary BCa (n = 9) | Five patients presented uptake in metastasis focus, indicating that HER2-negative primary BCa can generate HER2-positive metastases | Ulaner et al38 |
| 68Ga-HER2-nanobody; 53-174 MBq; PET | Early and metastatic breast carcinoma (n = 20) | Highest organ dose, respectively: urinary bladder wall, kidneys, liver, intestines; optimal image timing: 90 minutes PI; uptake in tumor lesions in 19 patients; clear tracer accumulation in metastatic lesions | Keyaerts et al39 |
| 89Zr-trastuzumab; 43.3-88.8 MBq; PET | Patients with BCa with at least 1 lesion determined by another imaging method (n = 12) | Optimal image timing: 5 days PI; liver was the dose-limiting organ; no adverse or clinically detectable pharmacological effects; uptake in at least 1 known lesion in 10 patients | Laforest et al40 |
| 68Ga-ABY-025; 215 MBq; PET | Metastatic BCa (n = 8) | Highest absorbed organ doses in the kidneys and liver, respectively; high dose of peptide gives low effective dose (5.6 mSv) than low dose (6.0 mSv); however, dose is much higher compared to 68Ga-DOTATATE and 68Ga-DOTATOC | Sandstrom et al41 |
| 68Ga-ABY-025; 212 MBq; PET | Metastatic BCa (n = 16) | Optimal image timing: 4 hours PI; PET imaging was accurate in identifying HER2-positive metastases, and PET SUV correlated with biopsy HER2-scores; noncompetitive binding with trastuzumab and pertuzumab | Sörensen et al42 |
| 89Zr-trastuzumab (37 MBq) + 18F-FDG; PET | Advanced BCa (n = 56) | The association of molecular imaging and metabolic imaging helped to identify lesions that do not respond to T-DM1 therapy and demonstrated that advanced HER2 BCa is highly heterogeneous disease | Gebhart et al43 |
| 111In-ABY-025; 142.6 MBq; SPECT | Recurrent metastatic breast cancer (n = 7) | High uptake in the kidneys, liver, and spleen; effective dose of 0.15 mSv/MBq; no drug-related adverse events; high-contrast HER2 images within 4 to 24 hours; visualization of metastases in the liver and brain | Sörensen et al44 |
| 177Lu-trastuzumab; 140.6-925 MBq; SPECT/Therapy | Early and advanced BCa (n = 10) | Optimal image timing: 5 or 7 days PI; uptake in primary and metastatic BCa lesion; accumulation in heart, liver, spleen, and nasopharynx; no leukopenia or liver toxicity was observed | Abbas et al45 |
Abbreviations: BCa, breast cancer; EGF, epidermal growth factor; HER2, human epidermal growth factor 2; PET, positron emission tomography; PI, postinjection; SC, subcutaneous; SPECT, single-photon emission computed tomography.
Intact Antibodies
Due to the long circulation time of antibodies in the blood, the choice of suitable radionuclides based on their physical half-lives (t1/2) is crucial. Zirconium-89 (89Zr) provides good positron emission tomography (PET) spatial resolution, as it decays via positron emission with a low average energy (Eβ+, average = 396 keV) and has a half-life of 3.27 days that matches the long biological half-life of antibodies.46,47
Frequently, the radiolabeling of intact antibodies with 89Zr is performed through the modification of a native lysine side chain with desferrioxamine-B (DFO).47,48 Nevertheless, it has been suggested that this chelator is not considered ideal for satisfying the coordination sphere of the Zr4+ cation, which can lead to the release of the radiometal from its chelator in rodent studies, leading to an accumulation in the bone.49 In rodents, it has been shown that 89Zr-oxalate and 89Zr-chloride show uptake in the bone (15%-20% injected dose per gram [ID/g]).49 However, most clinical studies showed that the dose absorbed by the bone/red bone marrow is almost negligible for 89Zr-mAb. For instance, Laforest et al24 showed that patients with BCa who were administered with 65 ± 18 MBq of 89Zr-trastuzumab had only 0.69 mGy/MBq in the red bone marrow. With the goal of creating more stable chelators for 89Zr, some groups have dedicated efforts to create new chelators as alternatives to DFO; however, none of these new chelators have yet to show significant improvement in their in vivo stability over DFO when conjugated with an antibody.50,51
The most widely used agents for imaging of HER2 are based on trastuzumab. In a preclinical study, Dijkers et al51 compared 89Zr-trastuzumab with 111In-trastuzumab, a radiopharmaceutical developed for antibody single-photon emission computed tomography (SPECT), and showed similar immunoreactivity and tumor uptake in xenograft models of BCa. Dijkers et al then conducted the first-in-human 89Zr-trastuzumab PET imaging clinical trial in patients with metastatic BCa.40 In this trial, 89Zr-trastuzumab showed excellent tumor uptake and visualization of HER2-positive metastatic lesions. These lesions were generally in agreement with available data from computed tomography (CT) and magnetic resonance imaging scans. It is well known that mAbs such as trastuzumab cannot cross an intact blood–brain barrier due to their large size, presenting a challenge in drug delivery for brain metastasis. However, Dijkers et al observed uptake in brain metastasis, likely due to the disruption of the blood–brain barrier at the site of the brain metastasis. During this study, no infusion-related reactions or adverse events were observed and the total radiation dose estimated was comparable to 2 abdominal CT scans (18 mSv).40
Similarly, a phase 0 study involving 12 patients with HER2-positive BCa confirmed that 89Zr-trastuzumab is safe and does not induce adverse effects. However, as 89Zr emits high-energy (909 keV) gamma rays associated with a high branching ratio (99.0%) coupled with the 511 keV gamma ray (22% branching ratio) for PET, dosimetry is an important concern.46 Laforest et al showed that 89Zr-trastuzumab had high residence times in several organs, mainly in the liver, kidney, and heart muscle. The liver was determined to be the critical organ, with a dose of 1.63 mSv/MBq (6.02 rad/mCi) due to the highest uptake of 89Zr-trastuzumab in this organ, as opposed to dose received from neighboring organs.24 An example of an image from this study is shown in Figure 1.
Figure 1.
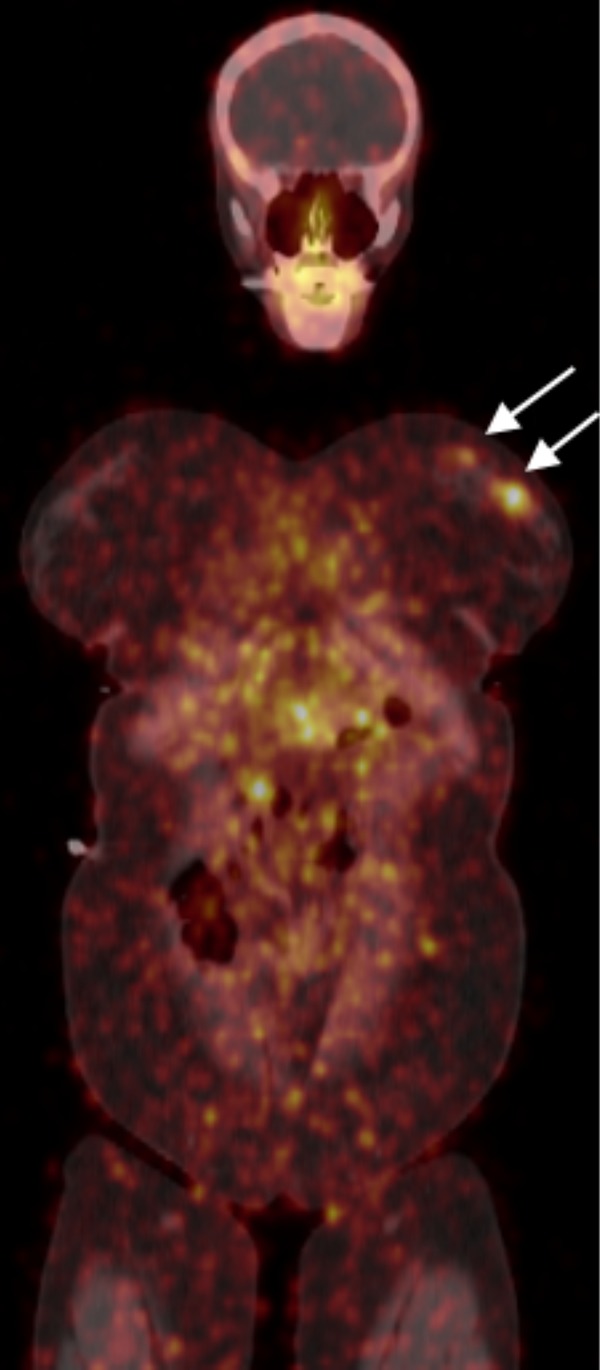
89Zr-trastuzumab imaging at 5 days postinjection in a patient with ER+/PR−/human epidermal growth factor 2 (HER2)+ multicentric primary breast cancer in the neoadjuvant setting.
89Zr-trastuzumab was also used to identify patients with HER2-positive metastatic BCa who were originally diagnosed with HER2-negative primary BCa.38 Ulaner et al evaluated 9 patients who received 185 MBq of 89Zr-trastuzumab. In this study, PET-CT visualized tumor foci, which were then biopsied and analyzed with IHC to confirm HER2 positivity. Five (56%) patients presented uptake of 89Zr-trastuzumab in metastatic foci, but only 2 were considered HER2-positive after the biopsy.38 Although this study involved a small sample population, the authors concluded that 89Zr-trastuzumab may help identify patients who will benefit from targeted therapy. In addition, they suggested that a larger sample population would be beneficial for future studies.38
Further, 89Zr-trastuzumab was studied in patients with advanced HER2-positive BCa to predict which patients are likely or unlikely to benefit from the use of T-DM1.43 The ZEPHIR study was a well-designed multicenter trial that enrolled patients from Belgium and the Netherlands who were eligible to receive T-DM1 for HER2-positive advanced disease.43 Gebhart et al evaluated 56 patients who received T-DM1 (3.6 mg/kg, every 3 weeks) using 8F-Fluodeoxyglucose(18F-FDG) and 89Zr-trastuzumab PET imaging. This study reported that 16 patients, who were previously diagnosed with HER2-positive metastasis by biopsy, did not present 89Zr-trastuzumab uptake in their lesions. The HER2-positive lesions were identified in 39 patients, and among them, 28 showed responses to the T-DM1 treatment.43
As mentioned previously, pertuzumab is currently being used in combination with trastuzumab.16 In nuclear medicine, this humanized mAb has been labeled with a variety of radiometals (Indium-111 [111In], Lutetium-177 [177Lu], and Copper-64 [64Cu])31,32,52–54 and has been proposed to be used as the imaging agent during monotherapy with trastuzumab33 because it binds to a different epitope (domain II of HER2) from that of trastuzumab (domain IV). Molecular biology studies have shown that the association of trastuzumab and pertuzumab promotes enhanced antitumor effect and increases binding affinity of both antibodies.15,33 A recent PET study has also complemented these studies showing that the tumor uptake of 89Zr-pertuzumab was increased in the presence of trastuzumab in BCa xenografts.33 Therefore, using 89Zr-pertuzumab as the imaging agent while treating with trastuzumab or T-DM1 may allow a more sensitive detection of HER2 without competing for binding.
In addition to BCa, HER2 is also overexpressed in other types of cancer, and thus, the advantages of molecular imaging can be extended to assess the HER2 status in other tumor types. Ovarian cancer (OVCa) has a lower incidence than BCa, with an estimated 22 440 new cases in 2017 in the United States. However, it is estimated that 62.74% of these new cases will result in death, making the mortality rate for this cancer higher than any other cancer of the female reproductive system.55,56 This mortality is attributed to late diagnosis when the disease has already advanced to late stages with metastasis in the peritoneal cavity.31,56
In animal models of OVCa, 64Cu-pertuzumab was used as an agent for imaging of HER2 and it was able to detect small peritoneal tumors in an orthotopic tumor model.31 Additionally, 89Zr-trastuzumab provided specific and high uptake in an HER2-positive model of OVCa in mice.50 These agents warrant potential investigation in patients with OVCa.
As shown, mAbs are being widely used in molecular imaging because they are specific for their targets and the established chemistry permits a convenient and fast production of these radiopharmaceuticals in high yields for preclinical and clinical studies. Nonetheless, their high molecular weight and consequently slow clearance make the best time for imaging at 4 to 7 days after injection due to the optimal tumor to background ratios achieved at these later time points. Thus, several strategies have been developed to design low-molecular-weight probes to accelerate rates of clearance and allow for imaging at earlier time points.
Antibodies Fragments and Other Small Molecules
With the goal to improve upon antibody pharmacokinetics without compromising affinity and specificity, several molecules have been bioengineered, including Fab and F(ab’)2 fragments (2 antigen-binding Fab portions linked together via disulfide bonds), affibodies, nanobodies, and minibodies.57 These relatively smaller scaffolds typically have shorter residence times in the bloodstream. Consequently, the imaging acquisition can be performed much sooner when compared to intact antibodies (hours versus days). Certainly, this approach represents greater convenience to the patient, as imaging can be performed within hours after being injected with the radiotracer. For example, nanobodies are considered the smallest naturally derived antigen-binding fragment (12-15 kDa), which consist of the variable domain of the heavy-chain portion of the immunoglobulin G.57 Recently, Keyaerts et al39 reported the safety, biodistribution, dosimetry, and tumor-targeting potential of a 68Ga-anti-HER2-nanobody in patients with breast carcinoma. Twenty patients were divided into 3 groups, and each group received a different dose of 68Ga-anti-HER2-nanobody (0.01-1 mg; 53-174 MBq). The biological half-life was estimated to be 1 hour, with the renal system being the main route for the elimination of the radiopharmaceutical. The organs that received the highest radiation doses were the urinary bladder wall (0.406 mGy/MBq), kidney (0.216 mGy/MBq), liver (0.0778 mGy/MBq), lower large intestine wall (0.0759 mGy/MBq), and upper large intestine wall (0.0619 mGy/MBq), respectively. The authors concluded that the use of 68Ga-anti-HER2-Nanobody is safe, up to 174 MBq, with no adverse effects.39
As mentioned previously, the mechanisms of resistance to trastuzumab are not well understood; however, Gallardo et al observed that trastuzumab resistance mechanisms are related to overexpression of EGFR and Insulin-like growth factor 1 receptor (IGF1R) in patients with HER2-positive carcinomas.58 Bispecific radioimmunoconjugates (bsRICs) that are capable of binding to HER2 and EGFR were developed for both therapy and diagnostic applications. These bsRICs are composed of the trastuzumab Fab (ligand for HER2) linked to EGF (ligand for EGFR) via a long spacer (polyethylene glycol [PEG24]).28,25 This long spacer was chosen to increase the blood residence time of the molecule (64Cu-NOTA-Fab-PEG24-EGF; NOTA: 1,4,7-triazacyclononane-triacetic acid), since small proteins such as Fabs can have rapid clearance that can lead to poor tumor uptake. 64Cu-NOTA-Fab-PEG24-EGF exhibited preserved specific binding to both EGFR and HER2 in vitro and high tumor uptake (28.9% ±7.37% ID/g at 48 hours postinjection [PI]) in mice bearing subcutaneous (SC) SKOV-3 (EGFRlow/HER2high) tumors.28,25
The pertuzumab Fab segment was also labeled with 64Cu in order to create a radiopharmaceutical that is able to detect changes in HER2 expression associated with response to trastuzumab treatment in BT-474 xenografted mice.23 Lam et al reported that 64Cu-NOTA-pertuzumab F(ab’)2 was able to detect a decrease in HER2 expression at 1 week after trastuzumab therapy. In addition, PET/CT images showed low uptake in normal organs except for the kidneys which presented the greatest uptake (52.4-65.6 %ID/g). The absorbed dose estimated for a human female adult was 1 mSv/MBq. The estimated whole-body equivalent dose was 0.015 mSv/MBq, which is 3.3 times less than the same probe labeled with 111In.23
Affibodies are medium-sized peptides (6.5 kDa) derived from a nonimmunoglobulin α-helix-based scaffold with similar or higher affinity than some mAb.59,42 ABY-025 is a second-generation affibody molecule with reduced nonspecific liver uptake that binds to domain III of the extracellular portion of HER2. Since trastuzumab and pertuzumab bind to domains IV and II, respectively, ABY-025 would promote a noncompetitive interaction, which may provide an important advantage for using this imaging agent in the presence of therapeutic concentrations of trastuzumab and/or pertuzumab.41,42,60 ABY-025 has been successfully labeled with 68Ga for PET41,42 and 111In for SPECT.44 Both modalities were able to discriminate HER2-positive from HER2-negative tumors in patients with metastatic BCa. The effective dose for a typical 200 MBq administration of 68Ga-ABY-025 was found to be between 5.6 and 6.0 mSv. The authors reported that this dose was lower than the effective dose for 200 MBq of 111In-ABY-025 (21 mSv) and an 18F-FDG PET scan, which usually delivers an effective dose of about 7 mSv.44
Another peptide that does not compete with trastuzumab for binding to HER2 is 99mTc-HYNIC-H6F.29,30 Li et al developed this novel SPECT imaging agent for similar reasons as the affibody probe—for noncompetitive binding with trastuzumab. In a preclinical model, 99mTc-HYNIC-H6F yielded rapid accumulation and relatively high uptake (2.47 ± 0.12 %ID/g at 30 minutes PI) in SC HER2-positive tumors (MDA-MB-453 cells) and low uptake (0.99 ± 0.19 %ID/g at 30 minutes PI) in HER2-negative tumors (MDA-MB-231 cells). As H6F binds to a different domain of HER2 (domain II), excess of trastuzumab did not block the binding of 99mTc-HYNIC-H6F nor did it inhibit its tumor accumulation.29
Another recent work evaluated a heptapeptide (LTVSPWY) conjugated with 2 different chelators (CGGG and CSSS) and labeled with 99mTc. In this study, Sabahnoo et al found that both peptides have high affinity (dissociation constant [KD] of 4.3 ± 0.8 nmol/L and 33.9 ± 9.7 nmol/L for 99mTc-CGGG-LTVSPWY and 99mTc-CSSS-LTVSPWY, respectively) to the extracellular domain II of HER2. The reduction in 99mTc-heptapeptide binding to SKOV-3 cells in the presence of trastuzumab showed that both peptides compete with trastuzumab. The higher uptake of 99mTc-CGGG-LTVSPWY in blood suggested that 99mTc was transchelated to plasma proteins. Both peptides showed similar tumor uptake (2.44 ± 1.12 %ID/g and 2.26 ± 1.28 %ID/g at 4 hours PI for 99mTc-CGGG-LTVSPWY and 99mTc-CSSS-LTVSPWY, respectively); however, the faster clearance of 99mTc-CSSS-LTVSPWY provided a higher tumor to normal organ ratio and better visualization of the tumor at 4 hours PI.30
The main advantage presented in using smaller scaffolds is the potential fast clearance with suitable affinity and specificity. Additionally, these molecules can pair well with shorter lived radioisotopes such as F-18 (t1/2 = 1.8 hours), thus reducing the dose to patients. In general, this fast clearance is also responsible for lower tumor uptake of the radiopharmaceuticals and an increased dose to the renal system.
In addition to smaller scaffolds, nanoparticles have been used as targeting vectors for HER2. Yamaguchi et al20 developed silica nanoparticles for multimodal imaging. They grafted hyperbranched polyamidoamine onto the surface of synthetic amorphous silica nanoparticles functionalized with indocyanine green (a fluorescence agent) and radiolabeled with 99mTc. Subsequently, anti-HER2 antibodies were conjugated to this nanoparticle to enable targeting of HER2-expressing cells. Although further studies will be necessary to increase in vivo stability, the authors conclude that these silica nanoparticles are promising for diagnostic and therapy applications. They also have the potential to be used as a drug delivery carrier for antitumor agents or β-emitting radioisotopes.20
Targeting of HER2 for Radionuclide Therapy
The term theranostic is defined as the integration of a diagnostic test with individualized therapy in the management of disease. Thus, a theranostic agent (or pair of imaging/therapeutic agents) can be used to identify those individuals who would benefit from a specific treatment, treat them, and monitor their responses to the treatment.61,62 Human epidermal growth factor 2 is an important target for theranostic use because the same or similar radiopharmaceutical can be used for either therapy or diagnosis, depending on the radionuclide used to label the molecule.
Therapy using radiopharmaceuticals is known as receptor radionuclide therapy or targeted radionuclide therapy, and the possibilities of developing new agents are promising. Antibodies used for targeted radionuclide therapy can be labeled with alpha, beta, and auger electron-emitting radionuclides. Similar to the diagnostic field of research, trastuzumab has been the focus for the development of HER2-targeted radiotherapeutics agents in the last decade. This antibody has been labeled with 177Lu, 64Cu, 111In,Thorium-227 (227Th), Rhenium-188 (188Re), Yttrium-90 (90Y), and Iodine-131 (131I).34–37,45,63–66
Iodine-131 is a β-emitter that is widely used in therapy owing to its cost-effective accessibility, long half-life (t1/2 = 8.1 days), and ease of radiolabeling and imaging capabilities due to its gamma emission.35 Radio-iodination can be damaging to mAbs; however, Kameswaran et al showed that 131I-trastuzumab preserved its immunoreactivity, affinity, and specific binding to HER2-positive cells (BT-474 and MDA-MB-453).35
Indium-111 is a radioisotope that can be used for imaging (SPECT) and therapy because of its auger electron emission. An auger electron is a low-energy electron with a very short path length and its cytotoxic effect only occurs when its nucleus decays in close proximity to the cell’s DNA. The high linear energy transfer of auger electrons enables In-111 to inflict lethal DNA damage.36,27
The use of a nuclear localizing signal (NLS) peptide has been proposed to achieve the efficient delivery of auger emitters into the tumor cell nucleus. Trastuzumab modified with NLS was labeled with 111In and its cytotoxicity was evaluated in SKBR3, MCF7 and HMEpC (human mammary epithelial cell) cells. Li et al found that 111In-trastuzumab-NLS was more cytotoxic than 111In-trastuzumab and its cytotoxicity was higher in the HER2-overexpressing SKBR3 cells than in the low-level HER2-expressing MCF7 and HMEpC cells. After a transcriptome analysis, Li et al determined that the DNA damage by auger electrons activated the nuclear factor κB pathway.36
Lutetium-177 is a radiolanthanide that has been demonstrated to be applicable for theranostic applications. It is a low-energy β-emitter (0.497 MeVmax) with tissue penetration up to 1.6 mm.65 Lutetium-177 is considered to be a viable alternative to 131I due to its lower energy γ-emission. Lutetium-177 has gamma energies of 208 keV (11% abundant) and 113 keV (6.4% abundant), while 131I has gamma energy of 364 keV (81% abundant). Thus, 177Lu has a considerable advantage in lower patient dosimetry and postpatient care.67
The same bsRICs labeled with 64Cu by Kwon et al were labeled with 177Lu and 111In, and the tumor growth inhibitory properties of 177Lu-DOTA-Fab-PEG24-EGF (DOTA: tetraazacyclododecane-1,4,7,10-tetraacetic acid) and 111In-DTPA-Fab-PEG24-EGF (DTPA: diethylenetriaminepentaacetic acid) were compared in mice implanted SC with trastuzumab-sensitive (HER2-positive MDA-MB-231/H2N) and acquired trastuzumab-resistant cells (HER2-positive TrR1—subclone of MDA-MB-231/H2N cells).25 The radiation-absorbed dose in the tumor for 177Lu-DOTA-Fab-PEG24-EGF was 9.3-fold higher, and thus, it was 1.3-fold more effective in inhibiting tumor growth than the 111In-labeled construct. The resistant tumor also responded to treatment with the 177Lu-labeled construct. The normal tissue toxicity studies for 177Lu-DOTA-Fab-PEG24-EGF showed that the ID of 3.7 and 11.1 MBq had no toxicity. However, when mice received a dose of 18.5 MBq, leukocyte and erythrocyte counts, hemoglobin, and hematocrit were significantly reduced compared to mice that received saline. Kwon et al determined that 11.1 MBq of 177Lu-DOTA-Fab-PEG24-EGF was the dose of the no observable adverse effect level.25
Abbas et al performed a preliminary clinical study with 177Lu-trastuzumab involving 10 patients in order to study the localization of the 177Lu-trastuzumab in primary and/or metastatic lesions and in the nontarget organs.45 177Lu-trastuzumab accumulated in the HER2-positive lesions, but no uptake was observed in the HER2-negative sites (as determined by IHC). Uptake in normal organs was observed in the heart, liver, spleen, and nasopharynx. In this study, the authors did not evaluate the radiation dose and toxicity to the white and red blood cells.45
Pertuzumab has also been labeled with 177Lu using the chelate isothiocyanate-benzyl-CHX-A″-DTPA. In a preclinical study using SKOV-3 OVCa xenografted Balb/c (nu/nu) mice, Persson et al found that 177Lu-DTPA-pertuzumab delivered a high dose (50.86 ± 5.57 Gy—activity injected of 7.3 MBq) to the tumor. The low bone uptake (0.07 ± 0.01 %ID/g at 14 days PI) suggested that this radiopharmaceutical was stable in vivo. The study group treated with 177Lu-pertuzumab showed a clear delay in tumor growth.52
Yttrium-90 is a pure β-emitting radioisotope with a path length of 5 mm and a high-energy β particle (2.296 MeV).68 This high energy also allows for the delivery of dose to adjacent tumor tissue that could be inaccessible because of tumor bulk, poor vascular supply, and/or tumor heterogeneity.68,69 Due to significant radiation dose that free 90Y can deliver to the bone, the development of chelators that improve upon the in vivo stability has been investigated. Price et al studied 2 acyclic chelators (H4octapa and CHX-A″-DTPA). This research demonstrated that both chelators were radiolabeled with fast kinetics without considerable heat (15 minutes at room temperature produced radiochemical yield >95%). Price et al determined that 90Y-CHX-A-DTPA-trastuzumab and 90Y-octapa-trastuzumab exhibited excellent in vitro and in vivo stability with high immunoreactivity. Also, both radiopharmaceuticals significantly inhibited tumor growth (tumor with 300 mm3 of volume after 36 days PI) compared to the control group (tumor with 1000 mm3 of volume after 36 days PI).34
Rhenium-188 is an attractive radionuclide for use in targeted radiotherapy due to its high-energy β particles (maximum energy = 2.11 MeV) and 155 keV gamma photons. Rhenium-188 has another advantage: It is conveniently produced from a transportable, alumina-based 188W/188Re generator, similar to a 99Mo/99mTc generator.37,70 Despite its attractive decay properties for targeted radiotherapy, 188Re has rarely been used for the development of targeted therapeutics for HER2-expressing tumors. In a recent study, Luo et al showed that 188Re-HYNIC-trastuzumab enhanced the cytotoxicity to tumor tissues to a level nearly 100-fold higher than that of the treatment with trastuzumab alone.37 Additionally, Altai et al conducted studies using an affibody (ZHER2: V2). Rhenium-188-ZHER2: V2 showed high affinity (6.4 ± 0.4 pico molar [pM].) and uptake in SKOV-3 xenografted tumors (14% ± 2 %ID/g at 1 hour PI) and rapid blood clearance. The dosimetry extrapolated to humans suggests that 188Re-ZHER2: V2 may provide an absorbed dose to tumors of more than 70 Gy.71 Future investigations in the clinical setting are needed to expand on this work.
An interesting approach combining targeted radiotherapy with brachytherapy is to inject radioisotopes, particularly β-emitters, directly in the tumor. For this purpose, gold nanoparticles (AuNP) were modified with trastuzumab and labeled with 177Lu26 and 111In.27 In previous work, these trastuzumab-AuNP were injected intravenously, resulting in moderate tumor uptake and high spleen uptake.72 Cai et al modified their strategy to inject these agents intratumorally. Both molecules showed significantly higher binding to the HER2-positive cells than the nontargeted nanoparticles (AuNP-177Lu and AuNP-111In). In addition, both were able to inhibit tumor growth in mice without apparent toxicity to the other tissues.27,26
In nuclear medicine, about 90% of the procedures performed worldwide are for diagnosis. Research in the development of new radiopharmaceuticals is also abundant for this application. Consequently, the vast majority of clinical trials being conducted are for diagnosis. However, efforts to develop new probes for therapy employing radiopharmaceuticals are currently expanding. These new investigations for therapy are producing promising results in preclinical studies and warrant investigation in clinical trials in the future.
Conclusions and Perspectives
Human epidermal growth factor 2 is an important target in oncology. The methods currently available to determine HER2 status are invasive and can show discrepancies due to tumor heterogeneity among other reasons. These inconsistent results may lead to suboptimal selection of patients for HER2-targeted therapy. In addition, not all lesions are readily accessible to be biopsied. In this scenario, molecular imaging offers an advantage because it is potentially a less invasive solution for the diagnosis of HER2-positive tumors. Therefore, radiopharmaceuticals targeting HER2 can help to identify patients who may benefit from HER2-targeted therapy and to monitor the change in HER2 status during therapy. Equally as important is the identification of patients who may not respond to HER2-targeted therapy so that alternative treatment options can be initiated early.
The high overexpression of HER2 in cancer cells relative to normal cells also makes HER2 a candidate for targeted radionuclide therapy, especially in cases where tumors are HER2 positive but resistant to some HER2-directed therapies. Improvements in radioisotope production have made radionuclides more available to more investigators, which contribute to the increase in biomedical research in this area. Human epidermal growth factor 2 is a relevant target for both diagnostic and therapeutic (theranostic) applications, and the development of molecules designed for HER2-directed therapy has been heavily explored in the last few years. With regard to the selection of probes for the clinical setting, Gebhart et al6 made an excellent point—the choice of the molecule will be guided by the desired application. While whole antibodies might be preferred for therapy approaches due to their longer residence time and consequently higher lethal radiation dose delivered to the tumor, smaller constructs such as nanobodies, peptides, and affibodies may be used as a diagnostic alternative to IHC or FISH. However, these probes may not necessarily reflect the delivery of antibody-based therapeutics; in this case, the antibody may be the best choice to be used as a diagnostic agent as well.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. [DOI] [PubMed] [Google Scholar]
- 2. Elizalde PV, Cordo Russo RI, Chervo MF, Schillaci R. ErbB-2 nuclear function in breast cancer growth, metastasis and resistance to therapy. Endocr Relat Cancer. 2016;23(12):T243–T257. [DOI] [PubMed] [Google Scholar]
- 3. Toss A, Venturelli M, Peterle C, Piacentini F, Cascinu S, Cortesi L. Molecular biomarkers for prediction of targeted therapy response in metastatic breast cancer: trick or treat? Int J Mol Sci. 2017;18(1):pii:E85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26(45):6469–6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19(13):3159–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gebhart G, Flamen P, De Vries EG, Jhaveri K, Wimana Z. Imaging diagnostic and therapeutic targets: human epidermal growth factor receptor 2. J Nucl Med. 2016;57(suppl):81S–8S. [DOI] [PubMed] [Google Scholar]
- 7. Asif HM, Sultana S, Ahmed S, Akhtar N, Tariq M. HER-2 positive breast cancer—a mini-review. Asian Pac J Cancer Prev. 2016;17(4):1609–1615. [DOI] [PubMed] [Google Scholar]
- 8. Schmidt KT, Chau CH, Price DK, Figg WD. Precision oncology medicine: the clinical relevance of patient specific biomarkers used to optimize cancer treatment. J Clin Pharmacol. 2016;56(12):1484–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gu G, Dustin D, Fuqua SA. Targeted therapy for breast cancer and molecular mechanisms of resistance to treatment. Curr Opin Pharmacol. 2016;31:97–103. [DOI] [PubMed] [Google Scholar]
- 10. Luque-Cabal M, García-Teijido P, Fernández-Pérez Y, Sánchez-Lorenzo L, Palacio-Vázquez I. Mechanisms behind the resistance to trastuzumab in HER2-amplified breast cancer and strategies to overcome it. Clin Med Insights Oncol. 2016;10(suppl 1):21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pareja F, Geyer FC, Marchiò C, Burke KA, Weigelt B, Reis-Filho JS. Triple-negative breast cancer: the importance of molecular and histologic subtyping, and recognition of low-grade variants. NPJ Breast Cancer. 2016;2:16036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nahta R. Molecular mechanisms of trastuzumab-based treatment in HER2-overexpressing breast cancer. ISRN Oncol. 2012;2012:428062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baselga J, Albanell J, Molina MA, Arribas J. Mechanism of action of trastuzumab and scientific update. Semin Oncol. 2001;28(5 suppl 16):4–11. [DOI] [PubMed] [Google Scholar]
- 14. Ponde NF, Lambertini M, De Azambuja E. Twenty years of anti-HER2 therapy-associated cardiotoxicity. ESMO Open. 2016;1(4):e000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009;69(24):9330–9336. [DOI] [PubMed] [Google Scholar]
- 16. Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krop I, Winer EP. Trastuzumab emtansine: a novel antibody–drug conjugate for HER2-positive breast cancer. J Oncol Pharm Pract. 2014;20(1):15–20. [DOI] [PubMed] [Google Scholar]
- 18. Phillips KA, Marshall DA, Haas JS, et al. Clinical practice patterns and cost-effectiveness of HER2 testing strategies in breast cancer patients. Cancer. 2009;115(22):5166–5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mathenge EG, Dean CA, Clements D, et al. Core needle biopsy of breast cancer tumors increases distant metastases in a mouse model. Neoplasia. 2014;16:950–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamaguchi H, Tsuchimochi M, Hayama K, Kawase T, Tsubokawa N. Dual-labeled near-infrared/(99 m)Tc imaging probes using PAMAM-coated silica nanoparticles for the imaging of HER2-expressing cancer cells. Int J Mol Sci. 2016;17(7):1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bose R, Kavuri SM, Searleman AC, et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013;3(2):224–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jia Y, Chen L, Jia Q, Dou X, Xu N, Liao DJ. The well-accepted notion that gene amplification contributes to increased expression still remains, after all these years, a reasonable but unproven assumption. J Carcinog. 2016;15:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lam K, Chan C, Reilly RM. Development and preclinical studies of 64Cu-NOTA-Pertuzumab F(ab’)2 for imaging changes in tumor HER2 expression associated with response to trastuzumab by PET/CT. MAbs. 2017;9(1):154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tinianow JN, Pandya DN, Pailloux SL, et al. Evaluation of a 3-hydroxypyridin-2-one (2,3-HOPO) based macrocyclic chelator for (89)Zr(4+) and its use for immunoPET imaging of HER2 positive model of ovarian carcinoma in mice. Theranostics. 2016;6(4):511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kwon LY, Scollard DA and Reilly RM. 64Cu-labeled trastuzumab Fab-PEG24-EGF radioimmunoconjugates bispecific for HER2 and EGFR: pharmacokinetics, biodistribution, and tumor imaging by PET in comparison to monospecific agents. Mol Pharm. 2017;14(2):492–501. [DOI] [PubMed] [Google Scholar]
- 26. Cai Z, Yook S, Lu Y, et al. Local radiation treatment of HER2-positive breast cancer using trastuzumab-modified gold nanoparticles labeled with 177Lu. Pharm Res. 2017;34(3):579–590. [DOI] [PubMed] [Google Scholar]
- 27. Cai Z, Chattopadhyay N, Yang K, et al. 111In-labeled trastuzumab-modified gold nanoparticles are cytotoxic in vitro to HER2-positive breast cancer cells and arrest tumor growth in vivo in athymic mice after intratumoral injection. Nucl Med Biol. 2016;43(12):818–826. [DOI] [PubMed] [Google Scholar]
- 28. Razumienko EJ, Chen JC, Cai Z, et al. Dual-receptor-targeted radioimmunotherapy of human breast cancer xenografts in athymic mice coexpressing HER2 and EGFR using 177Lu- or 111In-labeled bispecific radioimmunoconjugates. J Nucl Med. 2016;57(3):444–452. [DOI] [PubMed] [Google Scholar]
- 29. Li L, Wu Y, Wang Z, et al. SPECT/CT Imaging of the novel HER2-targeted peptide probe 99mTc-HYNIC-H6F in breast cancer mouse models. J Nucl Med. 2017;58(5):821–826. [DOI] [PubMed] [Google Scholar]
- 30. Sabahnoo H, Noaparast Z, Abedi SM, Hosseinimehr SJ. New small 99mTc-labeled peptides for HER2 receptor imaging. Eur J Med Chem. 2016;127:1012–1024. [DOI] [PubMed] [Google Scholar]
- 31. Jiang D, Im HJ, Sun H, et al. Radiolabeled pertuzumab for imaging of human epidermal growth factor receptor 2 expression in ovarian cancer. Eur J Nucl Med Mol Imaging. 2017;44(8):1296–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Persson M, Tolmachev V, Andersson K, Gedda L, Sandström M, Carlsson J. [(177)Lu]pertuzumab: experimental studies on targeting of HER-2 positive tumour cells. Eur J Nucl Med Mol Imaging. 2005;32(12):1457–1462. [DOI] [PubMed] [Google Scholar]
- 33. Marquez BV, Ikotun OF, Zheleznyak A, et al. Evaluation of (89)Zr-pertuzumab in breast cancer xenografts. Mol Pharm. 2014;11(11):3988–3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Price EW, Edwards KJ, Carnazza KE, et al. A comparative evaluation of the chelators H4octapa and CHX-A’’-DTPA with the therapeutic radiometal (90)Y. Nucl Med Biol. 2016;43(9):566–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kameswaran M, Gota V, Ambade R, Gupta S, Dash A. Preparation and preclinical evaluation of 131I-trastuzumab for breast cancer. J Labelled Comp Radiopharm. 2017;60(1):12–19. [DOI] [PubMed] [Google Scholar]
- 36. Li HK, Morokoshi Y, Daino K, et al. Transcriptomic signatures of auger electron radioimmunotherapy using nuclear targeting (111)In-trastuzumab for potential combination therapies. Cancer Biother Radiopharm. 2015;30(8):349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Luo TY, Cheng PC, Chiang PF, Chuang TW, Yeh CH, Lin WJ. 188Re-HYNIC-trastuzumab enhances the effect of apoptosis induced by trastuzumab in HER2-overexpressing breast cancer cells. Ann Nucl Med. 2015;29(1):52–62. [DOI] [PubMed] [Google Scholar]
- 38. Ulaner GA, Hyman DM, Ross DS, et al. Detection of HER2-positive metastases in patients with HER2-negative primary breast cancer using 89Zr-Trastuzumab PET/CT. J Nucl Med. 2016;57(10):1523–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Keyaerts M, Xavier C, Heemskerk J, et al. Phase I study of 68Ga-HER2-nanobody for PET/CT assessment of HER2 expression in breast carcinoma. J Nucl Med. 2016;57(1):27–33. [DOI] [PubMed] [Google Scholar]
- 40. Laforest R, Lapi SE, Oyama R, et al. [Zr89]Trastuzumab: evaluation of radiation dosimetry, safety, and optimal imaging parameters in women with HER2-positive breast cancer. Mol Imaging Biol. 2016;18(6):952–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sandstrom M, Lindskog K, Velikyan I, et al. Biodistribution and radiation dosimetry of the anti-HER2 affibody molecule 68Ga-ABY-025 in breast cancer patients. J Nucl Med. 2016;57(6):867–871. [DOI] [PubMed] [Google Scholar]
- 42. Sörensen J, Velikyan I, Sandberg D, et al. Measuring HER2-receptor expression in metastatic breast cancer using [68Ga]ABY-025 affibody PET/CT. Theranostics. 2016;6(2):262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gebhart G, Lamberts LE, Wimana Z, et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): the ZEPHIR trial. Ann Oncol. 2016;27(4):619–624. [DOI] [PubMed] [Google Scholar]
- 44. Sörensen J, Sandberg D, Sandstrom M, et al. First-in-human molecular imaging of HER2 expression in breast cancer metastases using the 111In-ABY-025 affibody molecule. J Nucl Med. 2014;55(5):730–735. [DOI] [PubMed] [Google Scholar]
- 45. Bhusari P, Vatsa R, Singh G, et al. Development of Lu-177-trastuzumab for radioimmunotherapy of HER2 expressing breast cancer and its feasibility assessment in breast cancer patients. Int J Cancer. 2017;140(4):938–947. [DOI] [PubMed] [Google Scholar]
- 46. Wooten A, Madrid E, Schweitzer G, et al. Routine production of 89Zr using an automated module. Appl Sci. 2013;3(3):593–613. [Google Scholar]
- 47. Wright BD, Lapi SE. Designing the magic bullet? The advancement of immuno-PET into clinical use. J Nucl Med. 2013;54(8):1171–1174. [DOI] [PubMed] [Google Scholar]
- 48. Vosjan MJ, Perk LR, Visser GW, et al. Conjugation and radiolabeling of monoclonal antibodies with zirconium-89 for PET imaging using the bifunctional chelate p-isothiocyanatobenzyl-desferrioxamine. Nat Protoc. 2010;5(4):739–743. [DOI] [PubMed] [Google Scholar]
- 49. Abou DS, Ku T, Smith-Jones PM. In vivo biodistribution and accumulation of 89Zr in mice. Nucl Med Biol. 2011;38(5):675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Boros E, Holland JP, Kenton N, Rotile N, Caravan P. Macrocycle-Based hydroxamate ligands for complexation and immunoconjugation of 89Zirconium for positron emission tomography (PET) imaging. Chempluschem. 2016;81(3):274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dijkers EC, Oude Munnink TH, Kosterink JG, et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther. 2010;87(5):586–592. [DOI] [PubMed] [Google Scholar]
- 52. Persson M, Gedda L, Lundqvist H, et al. [177Lu]pertuzumab: experimental therapy of HER-2-expressing xenografts. Cancer Res. 2007;67(1):326–331. [DOI] [PubMed] [Google Scholar]
- 53. Lam K, Scollard DA, Chan C, Levine MN, Reilly RM. Kit for the preparation of (111)In-labeled pertuzumab injection for imaging response of HER2-positive breast cancer to trastuzumab (Herceptin). Appl Radiat Isot. 2014;95C:135–142. [DOI] [PubMed] [Google Scholar]
- 54. Lam K, Chan C, Done SJ, Levine MN, Reilly RM. Preclinical pharmacokinetics, biodistribution, radiation dosimetry and acute toxicity studies required for regulatory approval of a clinical trial application for a phase I/II clinical trial of (111)In-BzDTPA-pertuzumab. Nucl Med Biol. 2015;42(2):78–84. [DOI] [PubMed] [Google Scholar]
- 55. American Cancer Society. What are the key statistics about ovarian cancer? https://www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html. Accessed 28 December 2017.
- 56. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 57. Chakravarty R, Goel S, Cai W. Nanobody: the “magic bullet” for molecular imaging? Theranostics. 2014;4(4):386–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gallardo A, Lerma E, Escuin D, et al. Increased signalling of EGFR and IGF1 R, and deregulation of PTEN/PI3K/Akt pathway are related with trastuzumab resistance in HER2 breast carcinomas. Br J Cancer. 2012;106 (8):1367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Minelic DE. Antibody Engineering: Optimizing the delivery vehicle In: Reilly RM. ed. Monoclonal Antibody and Peptide-Targeted Radiotherapy of Cancer. Hoboken, NJ: John Wiley & Sons; 2010:1–38. [Google Scholar]
- 60. Sandberg D, Tolmachev V, Velikyan I, et al. Intra-image referencing for simplified assessment of HER2-expression in breast cancer metastases using the Affibody molecule ABY-025 with PET and SPECT. Eur J Nucl Med Mol Imaging. 2017;44(8):1337–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Taïeb D, Hicks RJ, Pacak K. Nuclear medicine in cancer theranostics: beyond the target. J Nucl Med. 2016;57(11):1659–1660. [DOI] [PubMed] [Google Scholar]
- 62. Kelkar SS, Reineke TM. Theranostics: combining imaging and therapy. Bioconjug Chem. 2011;22(10):1879–1903. [DOI] [PubMed] [Google Scholar]
- 63. Rasaneh S, Rajabi H, Babaei MH, Daha FJ, Salouti M. Radiolabeling of trastuzumab with 177Lu via DOTA, a new radiopharmaceutical for radioimmunotherapy of breast cancer. Nucl Med Biol. 2009;36(4):363–369. [DOI] [PubMed] [Google Scholar]
- 64. Rasaneh S, Rajabi H, Babaei MH, Daha FJ. 177Lu labeling of Herceptin and preclinical validation as a new radiopharmaceutical for radioimmunotherapy of breast cancer. Nucl Med Biol. 2010;37(8):949–955. [DOI] [PubMed] [Google Scholar]
- 65. Yong KJ, Milenic DE, Baidoo KE, et al. Mechanisms of cell killing response from low linear energy transfer (LET) radiation originating from (177)Lu radioimmunotherapy targeting disseminated intraperitoneal tumor xenografts. Int J Mol Sci. 2016;17(5).pii:E736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Abbas N, Bruland OS, Brevik EM, et al. Preclinical evaluation of 227Th-labeled and 177Lu-labeled trastuzumab in mice with HER-2-positive ovarian cancer xenografts. Nucl Med Commun. 2012;33(8):838–847. [DOI] [PubMed] [Google Scholar]
- 67. Pillai MR, Chakraborty S, Das T, Venkatesh M, Ramamoorthy N. Production logistics of 177Lu for radionuclide therapy. Appl Radiat Isot. 2003;59(2-3):109–118. [DOI] [PubMed] [Google Scholar]
- 68. Rizzieri D. Zevalin® (ibritumomab tiuxetan): after more than a decade of treatment experience, what have we learned? Crit Rev Oncol Hematol. 2016;105:5–17. [DOI] [PubMed] [Google Scholar]
- 69. Brady D, O’Sullivan JM, Prise KM. What is the role of the bystander response in radionuclide therapies? Front Oncol. 2013;3:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ogawa K, Kawashima H, Kinuya S, et al. Preparation and evaluation of 186/188Re-labeled antibody (A7) for radioimmunotherapy with rhenium(I) tricarbonyl core as a chelate site. Ann Nucl Med. 2009;23(10):843–848. [DOI] [PubMed] [Google Scholar]
- 71. Altai M, Wallberg H, Honarvar H, et al. 188Re-ZHER2: V2, a promising affibody-based targeting agent against HER2-expressing tumors: preclinical assessment. J Nucl Med. 2014;55(11):1842–1848. [DOI] [PubMed] [Google Scholar]
- 72. Chattopadhyay N, Fonge H, Cai Z, et al. Role of antibody-mediated tumor targeting and route of administration in nanoparticle tumor accumulation in vivo. Mol Pharm. 2012;9(8):2168–2179. [DOI] [PubMed] [Google Scholar]


