Abstract
Introduction
Identification of patients with an increased risk of high defibrillation thresholds (DFTs) is important in planning implantable cardioverter-defibrillator (ICD) procedures. Clinical observations have suggested that patients with methamphetamine cardiomyopathy (MACMP) have significantly elevated defibrillation thresholds. We hypothesized that MACMP patients would have higher DFT thresholds than controls and would require procedural changes during ICD implantation to accommodate higher thresholds.
Methods
We identified consecutive patients with MACMP undergoing ICD implantation at the academic center from 2003 to 2007. We then compared DFTs against age-and sex-matched controls.
Results
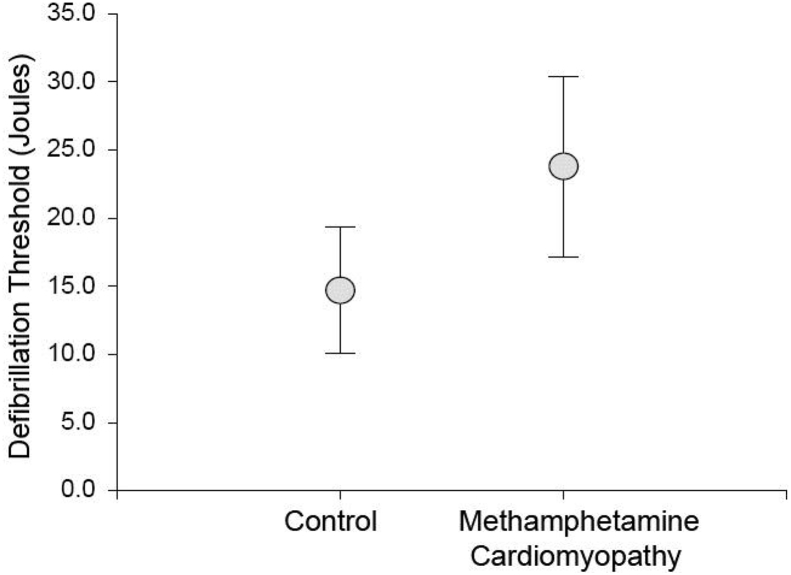
The MACMP (n = 10) group showed significantly increased DFT thresholds (23.7 ± 6.7 J) compared with age and sex-matched controls (14.5 ± 4.6 J, p < 0.005). Additionally, patients with MACMP had evidence of more severe congestive heart failure, with increased B-type natrieutic protein (BNP) levels (1173 ± 784 vs 260 ± 349, p = 0.02) and decreased left ventricular ejection fraction (LVEF) (17.8 ± 9.4 vs 35.9 ± 15.2, p = 0.02). MACMP patients required high output devices than controls (50% versus 0%, p = 0.03). Differences between groups remained significant despite adjusting for LVEF.
Conclusions
Planning for ICD implantation should take into consideration a history of methamphetamine abuse, mandating DFT testing and empiric consideration of high output devices for such patients.
Keywords: Methamphetamine cardiomyopathy, Implantable cardioverter-defibrillatior, Defibrillation threshold testing, B-type natriuretic peptide, Ejection fraction
1. Introduction
Despite advances in implantable cardioverter-defibrillation (ICD) technology, elevated defibrillation thresholds (DFTs) occur in over 6% of patients undergoing ICD implantation [1]. High thresholds present significant challenges to achieve adequate defibrillation efficacy, often requiring change in lead position [2], reversal of shock polarity [3], reprogramming waveform duration [4], use of a high-output energy device [3], or addition of superior vena cava or subcutaneous array [5], [6] to achieve a satisfactory defibrillation threshold. Thus, preoperative identification of patients at risk for elevated DFTs can facilitate ICD procedure planning.
Methamphetamine, a growing drug of abuse [7] is associated with increased non-ischemic dilated cardiomyopathy [8], [9], [10] and sudden death [11], [12]. Methamphetamine abuse is associated with a number of cardiac pathologic changes [13] including hypertrophy, myocardial disarray, and fibrosis [14]. An increasing number of these patients are referred for ICD implantation for primary and secondary indications.
Clinically, we have observed a high prevalence of elevated DFTs in these patients. We hypothesized that patients with methamphetamine cardiomyopathy (MACMP) have higher defibrillation thresholds than controls. Furthermore, we hypothesized that these patients more frequently require significant changes to ICD implantation procedure, including use of high energy devices to provide effective defibrillation therapy.
2. Methods
This study is a retrospective, case-control study, performed with the approval of the local Institutional Review Board.
2.1. Data collection
We identified 10 patients from the medical record with documented methamphetamine cardiomyopathy who underwent first ICD implantation at the university hospital between November 2003 to December 2007. We then evaluated 79 patients (18 women and 61 men, mean age 59.60 ± 5.13) who underwent first ICD implantation without MACMP between December 2005 and November 2007 as potential control patients, and matched cases versus controls for age and sex. Patient characteristic including age, sex, body surface area, electrocardiogram (ECG) parameters, B-type natriuretic peptide (BNP), left ventricular ejection fraction (LVEF), DFT, and use of high output ICD during device implantation for both case and control groups were recorded and compared.
2.2. Defibrillation threshold testing
All patients included in the study were subjected to clinical DFT testing during ICD implantation. DFT was measured using a step-down protocol (i.e. step-wise reduction of Direct-Current (DC) shock strength during serial inductions of ventricular fibrillation (VF), until the ICD shock fails to cardiovert VF).
2.3. Events during ICD implantation
We reviewed the operative reports from all patients to determine what impact higher DFTs had upon ICD implantation procedures. Events recorded included change in defibrillation vector, use of high energy ICD pulse generator (≥35 J delivered), and use of subcutaneous array.
2.3.1. Evaluation for possible confounders
We evaluated the correlation between DFT and potential confounders for using the cohort of patients in the study. We then adjusted the comparison of methamphetamine versus matched control patients to determine if the difference remained significant.
2.4. Statistical analysis
Continuous data are represented as mean ± standard deviation (SD). The characteristics of patients in both groups were compared through use of paired t-test for numerical data and the McNemar's test for nominal data. Proportions were compared using Fisher's exact test. Analysis of covariance was used to adjust for other sources of variation to DFT. Linear regression was used to evaluate DFT versus LVEF and log (BNP). All tests were two sided and differences were considered statistically significant if the null hypothesis could be rejected with more than 95% confidence (P < 0.05) Statistical analysis was performed using NCSS 2007 (Kaysville, UT).
3. Results
Compared to age- and sex-matched controls, the methamphetamine group (n = 10, age 44.2 years, 7 male) showed significantly elevated BNP (Log BNP 2.88 ± 0.49 vs 2.00 ± 0.72, p = 0.02) and reduced LVEF (17.9 ± 9.4 vs 35.9 ± 15.2, p = 0.02) at baseline prior to device implantation (Table 1). There were no significant differences in body surface area and bi-ventricular ICD implantation or ECG indices including: heart rate, PR, QRS and QTc intervals. Overall, both groups were well matched in use of amiodarone, class I anti-arrhythmics, beta-blockers, and ACE inhibitors.
Table 1.
Characteristics of patients using meth vs. patients not using meth.
| Characteristic | Patients using meth (N = 10) | Patients not using meth (N = 10) | P Value |
|---|---|---|---|
| Age at DFT, yr. | 44.2 ± 8.4 | 44.2 ± 9.3 | Matched |
| Male Gender, n(%) | 7 (70%) | 6 (60%) | NS |
| Defibrillation Threshold, J | 23.7 ± 6.7 | 14.7 ± 4.6 | <0.005 |
| PR Interval, ms | 189 ± 39 | 154 ± 38 | 0.06 |
| QRS Interval, ms | 122 ± 31 | 109 ± 31 | 0.37 |
| QTc Interval, ms | 470 ± 41 | 446 ± 36 | 0.26 |
| Ventricular rate | 93 ± 28 | 82 ± 14 | 0.3 |
| Ejection Fraction, % | 17.8 ± 9.4 | 35.9 ± 15.2 | 0.02 |
| BNP | 1173 ± 784 | 260 ± 349 | 0.02 |
| - Log (BNP) | 2.88 ± 0.49 | 2.00 ± 0.72 | 0.02 |
| Body Surface Area, m2 | 1.82 ± 0.14 | 1.98 ± 0.38 | 0.18 |
| Biventricular ICD, n(%) | 4 (40%) | 2 (20%) | 0.62 |
| Amiodarone, n(%) | 0 (0%) | 1 (10%) | NS |
| Class I AA, n(%) | 0 (0%) | 0 (0%) | NS |
| ACEi or ARB, n(%) | 8 (80%) | 9 (90%) | NS |
| Beta-blockers, n(%) | 7 (70%) | 8 (80%) | NS |
3.1. Elevated defibrillation threshold and effects on ICD procedure
The mean DFT of patients with history of methamphetamine was significantly greater than controls (23.7 ± 6.7 vs 14.7 ± 4.6, p < 0.005, Fig. 1). Due to their elevated DFT, a total of 5 patients (50%) received a high energy ICD pulse generator. In contrast, none (p = 0.033) of controls required high energy devices to achieve an acceptable safety margin.
Fig. 1.
Comparison of defibrillation thresholds (DFTs) between control and methamphetamine cardiomyopathy (MACMP) patients is shown. Significantly higher DFTs were found in the MACMP population (23.7 ± 6.7 vs 14.7 ± 4.6, p < 0.005).
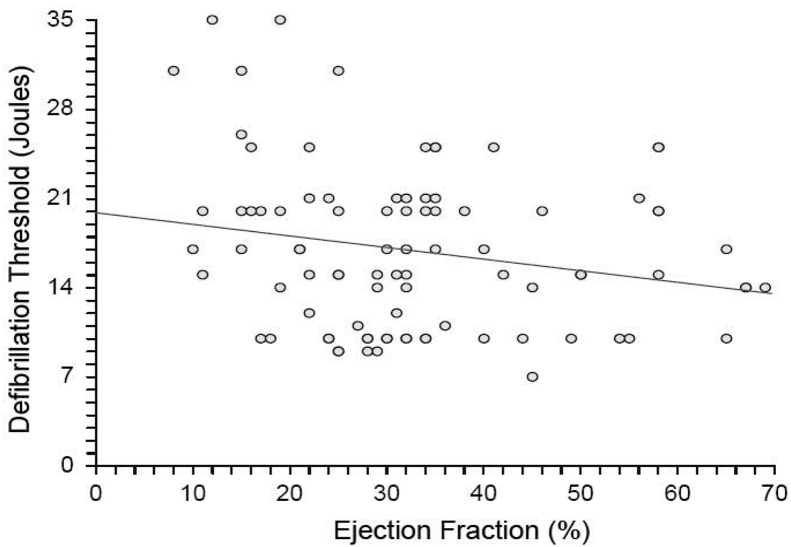
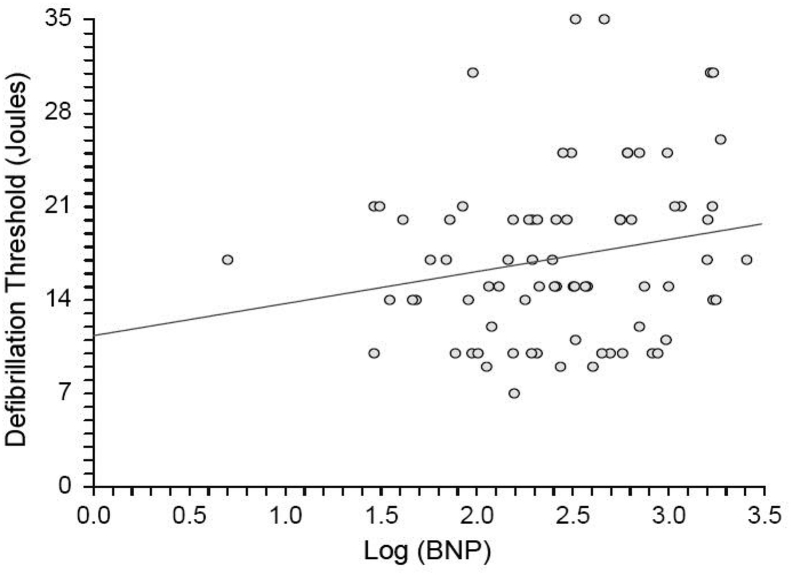
3.2. Evaluation of DFT versus LVEF and log (BNP)
Because there were significant differences between MACMP and control patients in LVEF and log (BNP), we attempted to determine if differences in DFT persisted after adjusting for these variables. Interestingly, there was no significant relationship between DFT and LVEF for the entire study population (Fig. 2), including methamphetamine patients. There was a weak relationship between DFT and log (BNP) (Fig. 3). However, this accounted for only a small portion of the observed variability in DFT, given the low slope of the correlation and the low R-squared value.
Fig. 2.
There was a significant relationship between left ventricular ejection fraction (LVEF) and defibrillation threshold (DFT) for the population (DFT = 19.8689–0.0910 (LVEF), p = 0.045). However, this relationship accounted for only a fraction of the variation, as R-squared was 0.0453.
Fig. 3.
No relationship was found between log (B-type natriuretic peptide (BNP)) and defibrillation threshold (DFT) for the population (DFT = 11.3305 + 2.3975 (log(BNP)), p = 0.08). The correlation was poor, with R-squared of 0.041.
Differences in DFT between patients with and without MACMP persisted even after comparisons were adjusted for log (BNP). Further adjustment for differences in LVEF also did not affect the significance of the results.
4. Discussion
The findings of this study confirm the hypothesis that patients with MACMP have significantly elevated DFTs at ICD implantation. This represents an important addition to the list of subgroups presenting for device therapy already shown to have high DFTs, including patients with cocaine abuse [15], hypertrophic cardiomyopathy [16], [17], and amiodarone therapy [1], [18], [19], [20], among others. Although, there is some debate about routine DFT testing for all ICD implantation procedures [1], [21] due to complications including prolonged hypotension, hypoperfusion and circulatory arrest, thromboembolic episodes, and use of heavier sedation [21]. Also, recent randomized controlled study among 145 patients with heart failure and severe LV dysfunction revealed no significant differences in perioperative complications, failed appropriate shocks, or arrhythmic death, among those with and without intraoperative DFT [22]. However, there are certain group of high risk patients who requires DFT testing to assure effective defibrillation therapy, based on the fact that a standard 31 J ICD would be unable to unable to provide a 10 J safety margin to a majority of patients with high defibrillation threshold including our MACMP population (average DFT 24 J).
While MACMP patients in our study had evidence of more advanced congestive heart failure, adjusting for these variables did not affect the significance of the results. This is not surprising, given the variable findings within the literature on the effect of left ventricular dysfunction on DFT. In particular, studies by Shukla et al. [23] and Lubinski et al. [20] both found that low LVEF predicted higher DFTs. In contrast, Epistein et al. [18] found no clinical characteristic other than amiodarone use could consistently predict elevated DFT. The mechanism behind MACMP is multifactorial and may be attributed to direct toxicity, catecholamine excess, coronary vasospasm, increases in reactive oxygen species (ROS), mitochondrial injury, and changes in myocardial metabolism. The above mentioned etiologies along with degree of LV fibrosis and autonomic dysregulation due to sympathomimetic substance abuse may be responsible for increased defibrillation energy requirements.
It is interesting to note that the findings of this study are in close agreement with the findings of Chen et al. [15] regarding the effects of cocaine, also a powerful sympathomimetic agent, on DFT thresholds. Previous work, although conflicting, has shown the effects of circulating catecholamines on DFT [24], [25]. Future work is required to determine if changes in autonomic function underlie the electrophysiologic mechanisms of VF reinitiation following failed defibrillation.
Our study showed significant changes to the ICD implantation procedure for a high risk population. This has important implications for procedure planning. Given the high cost ICD pulse generators, it may be economically advantageous to empirically select high output devices for this population rather than a standard output ICD, given that unsuccessful devices are not reusable for other patients, and must be recycled. However, prospective cost-effectiveness studies are required to definitively answer this question.
5. Conclusions
Planning for ICD implantation should take into consideration a history of methamphetamine abuse, mandating DFT testing and consideration of empiric high energy devices for such patients.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Russo A.M., Sauer W., Gerstenfeld E.P., Hsia H.H., Lin D., Cooper J.M. Defibrillation threshold testing: is it really necessary at the time of implantable cardioverter-defibrillator insertion? Heart Rhythm. 2005;2:456–461. doi: 10.1016/j.hrthm.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 2.Tang A.S., Hendry P., Goldstein W., Green M.S., Luce M. Nonthoracotomy implantation of cardioverter defibrillators: preliminary experience with a defibrillation lead placed at the right ventricular outflow tract. Pacing Clin Electrophysiol. 1996;19:960–964. doi: 10.1111/j.1540-8159.1996.tb03393.x. [DOI] [PubMed] [Google Scholar]
- 3.Brooks R., Torchiana D., Vlahakes G.J., Ruskin J.N., McGovern B.A., Garan H. Successful implantation of cardioverter-defibrillator systems in patients with elevated defibrillation thresholds. J Am Coll Cardiol. 1993;22:569–574. doi: 10.1016/0735-1097(93)90066-a. [DOI] [PubMed] [Google Scholar]
- 4.Keane D., Aweh N., Hynes B., Sheahan R.G., Cripps T., Bashir Y. Achieving sufficient safety margins with fixed duration waveforms and the use of multiple time constants. Pacing Clin Electrophysiol. 2007;30:596–602. doi: 10.1111/j.1540-8159.2007.00718.x. [DOI] [PubMed] [Google Scholar]
- 5.Gradaus R., Block M., Seidl K., Brunn J., Isgro F., Hammel D. Defibrillation efficacy comparing a subcutaneous array electrode versus an “active can” implantable cardioverter defibrillator and a subcutaneous array electrode in addition to an “active can” implantable cardioverter defibrillator: results from active can versus array trials I and II. J Cardiovasc Electrophysiol. 2001;12:921–927. doi: 10.1046/j.1540-8167.2001.00921.x. [DOI] [PubMed] [Google Scholar]
- 6.Gold M., Val-Mejias J., Leman R.B., Tummala R., Goyal S., Kluger J. Optimization of superior vena cava coil position and usage for transvenous defibrillation. Heart Rhythm. 2008;5:394–399. doi: 10.1016/j.hrthm.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Albertson T.E., Derlet R.W., Van Hoozen B.E. Methamphetamine and the expanding complications of amphetamines. West J Med. 1999;170:214–219. [PMC free article] [PubMed] [Google Scholar]
- 8.Hong R., Matsuyama E., Nur K. Cardiomyopathy associated with the smoking of crystal methamphetamine. Jama. 1991;265:1152–1154. [PubMed] [Google Scholar]
- 9.Wijetunga M., Seto T., Lindsay J., Schatz I. Crystal methamphetamine-associated cardiomyopathy: tip of the iceberg? J Toxicol Clin Toxicol. 2003;41:981–986. doi: 10.1081/clt-120026521. [DOI] [PubMed] [Google Scholar]
- 10.Yeo K.K., Wijetunga M., Ito H., Efird J.T., Tay K., Seto T.B. The association of methamphetamine use and cardiomyopathy in young patients. Am J Med. 2007;120:165–171. doi: 10.1016/j.amjmed.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 11.Inoue H., Ikeda N., Kudo K., Ishida T., Terada M., Matoba R. Methamphetamine-related sudden death with a concentration which was of a 'toxic level'. Leg Med (Tokyo) 2006;8:150–155. doi: 10.1016/j.legalmed.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Nishida N., Ikeda N., Kudo K., Esaki R. Sudden unexpected death of a methamphetamine abuser with cardiopulmonary abnormalities: a case report. Med Sci Law. 2003;43:267–271. doi: 10.1258/rsmmsl.43.3.267. [DOI] [PubMed] [Google Scholar]
- 13.Kaye S., McKetin R., Duflou J., Darke S. Methamphetamine and cardiovascular pathology: a review of the evidence. Addiction. 2007;102:1204–1211. doi: 10.1111/j.1360-0443.2007.01874.x. [DOI] [PubMed] [Google Scholar]
- 14.Matoba R. Cardiac lesions in methamphetamine abusers. Nihon Hoigaku Zasshi. 2001;55:321–330. [PubMed] [Google Scholar]
- 15.Chen J., Naseem R.H., Obel O., Joglar J.A. Habitual cocaine use is associated with high defibrillation threshold during ICD implantation. J Cardiovasc Electrophysiol. 2007;18:722–725. doi: 10.1111/j.1540-8167.2007.00834.x. [DOI] [PubMed] [Google Scholar]
- 16.Almquist A.K., Montgomery J.V., Haas T.S., Maron B.J. Cardioverter-defibrillator implantation in high-risk patients with hypertrophic cardiomyopathy. Heart Rhythm. 2005;2:814–819. doi: 10.1016/j.hrthm.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Jastrzebski M., Czarnecka D., Bacior B., Petkow-Dimitrow P., Wilczek R., Kawecka-Jaszcz K. Massive myocardial hypertrophy in hypertrophic cardiomyopathy: a risk factor for sudden death and high defibrillation threshold during cardioverter-defibrillator implantation. Kardiol Pol. 2005;63:191–195. discussion 196. [PubMed] [Google Scholar]
- 18.Epstein A.E., Ellenbogen K.A., Kirk K.A., Kay G.N., Dailey S.M., Plumb V.J. Clinical characteristics and outcome of patients with high defibrillation thresholds. A multicenter study. Circulation. 1992;86:1206–1216. doi: 10.1161/01.cir.86.4.1206. [DOI] [PubMed] [Google Scholar]
- 19.Hohnloser S.H., Dorian P., Roberts R., Gent M., Israel C.W., Fain E. Effect of amiodarone and sotalol on ventricular defibrillation threshold: the optimal pharmacological therapy in cardioverter defibrillator patients (OPTIC) trial. Circulation. 2006;114:104–109. doi: 10.1161/CIRCULATIONAHA.106.618421. [DOI] [PubMed] [Google Scholar]
- 20.Lubinski A., Lewicka-Nowak E., Zienciuk A., Krolak T., Kempa M., Pazdyga A. Clinical predictors of defibrillation threshold in patients with implantable cardioverter-defibrillators. Kardiol Pol. 2005;62:317–328. [PubMed] [Google Scholar]
- 21.Gula L.J., Massel D., Krahn A.D., Yee R., Skanes A.C., Klein G.J. Is defibrillation testing still necessary? A decision analysis and Markov model. J Cardiovasc Electrophysiol. 2008;19:400–405. doi: 10.1111/j.1540-8167.2007.01095.x. [DOI] [PubMed] [Google Scholar]
- 22.Healey J.S., Gula L.J., Birnie D.H., Sterns L., Connolly S.J., Sapp J. A randomized-controlled pilot study comparing ICD implantation with and without intraoperative defibrillation testing in patients with heart failure and severe left ventricular dysfunction: a substudy of the RAFT trial. J Cardiovasc Electrophysiol. 2012;23:1313–1316. doi: 10.1111/j.1540-8167.2012.02393.x. [DOI] [PubMed] [Google Scholar]
- 23.Shukla H.H., Flaker G.C., Jayam V., Roberts D. High defibrillation thresholds in transvenous biphasic implantable defibrillators: clinical predictors and prognostic implications. Pacing Clin Electrophysiol. 2003;26:44–48. doi: 10.1046/j.1460-9592.2003.00148.x. [DOI] [PubMed] [Google Scholar]
- 24.Kalus J.S., White C.M., Caron M.F., Guertin D., McBride B.F., Kluger J. The impact of catecholamines on defibrillation threshold in patients with implanted cardioverter defibrillators. Pacing Clin Electrophysiol. 2005;28:1147–1156. doi: 10.1111/j.1540-8159.2005.09484.x. [DOI] [PubMed] [Google Scholar]
- 25.Sousa J., Kou W., Calkins H., Rosenheck S., Kadish A., Morady F. Effect of epinephrine on the efficacy of the internal cardioverter-defibrillator. Am J Cardiol. 1992;69:509–512. doi: 10.1016/0002-9149(92)90995-b. [DOI] [PubMed] [Google Scholar]