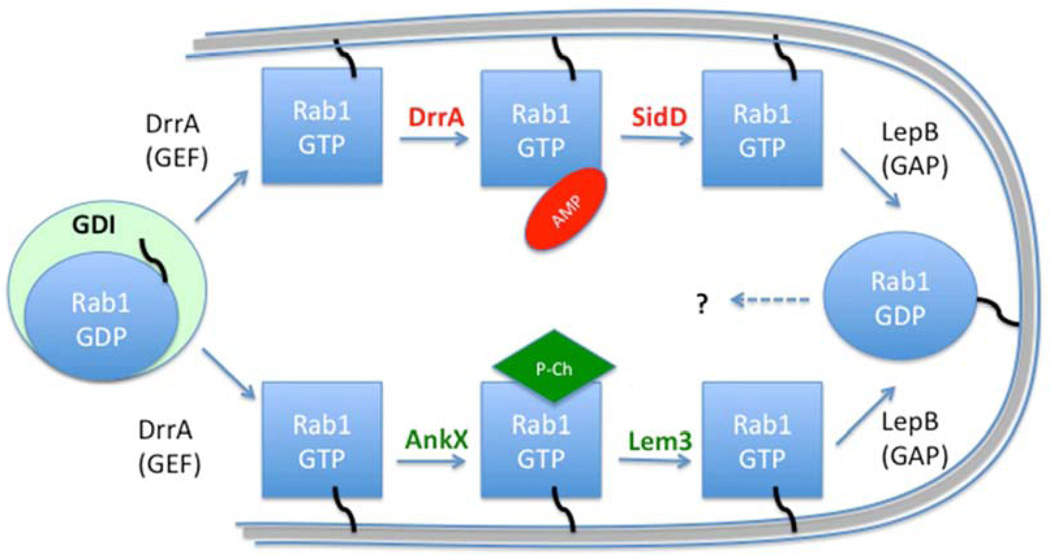
Figure 2. Legionella uses AMPylation and phosphocholination to regulate the Rab1 GTPase. Rab1 is sequestered in the cytoplasm by a guanine dissociation inhibitor (GDI).
Upon infection, Rab1 is recruited to the Legionella-containing vacuole (LCV) membrane followed by activation by the Legionella GEF DrrA. It is then modified by either AMPylation or phosphocholination by the bacterial effectors DrrA or AnkX, respectively. The modified form of Rab1 cannot interact with host GEFs and GAPs. The PTMs on activated GTP-bound Rab1 can be reversed by deAMPylation or dephosphocholination by the effectors SidD or Lem3, respectively. The GTP-bound Rab1 can then be inactivated by the Legionella GAP LepB.