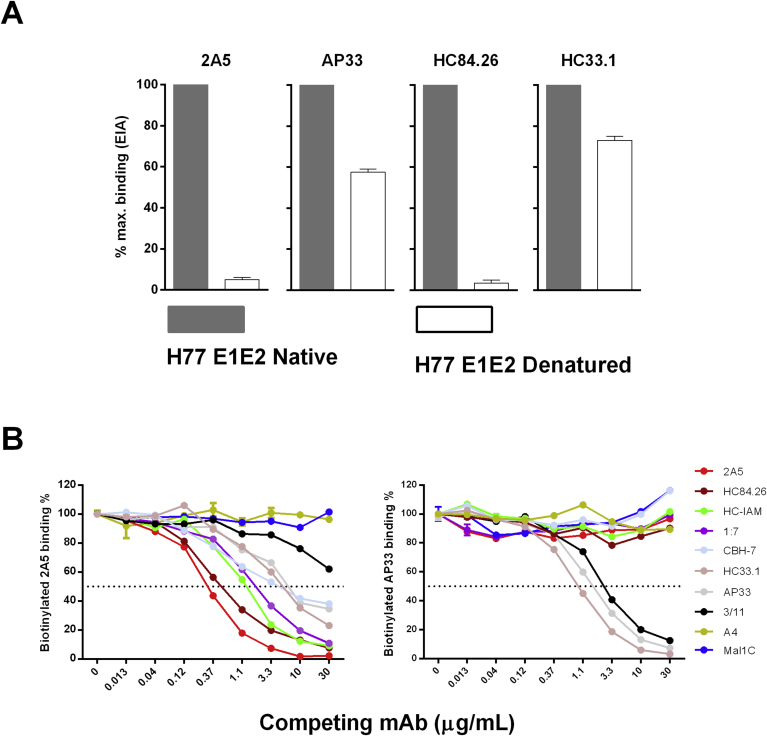
Fig. 7.
Recognition by mAb 2A5 is conformation dependent. (A) To reveal whether mAb 2A5 recognizes a linear or a conformational epitope, mAbs 2A5, AP33, HC84.26 or HC33.1 were incubated at 5 μg/mL with native as well as denatured full-length H77c E1E2 cell lysates. Bound antibodies were detected with HRP-conjugated secondary antibodies and the signal was normalized to that obtained with the native protein. (B) To analyze whether 2A5 competes with other mAb, a competition EIA was performed wherein anti-E2 (AP33, HC33.1, HC84.26, HC-1AM, CBH-7, 1:7 and 3/11) and anti-E1 (A4) mAbs were used as competing antibodies for binding to the E1E2 protein of the H77c isolate. Three-fold serial dilutions of competing mAbs were used starting at 30 μg/mL. The binding of biotinylated versions of mAb 2A5 (left panel) and AP33 (right panel) was measured using HRP-conjugated streptavidin. Mal1C is an irrelevant antibody that was included as negative control. All conditions were performed in duplicate.