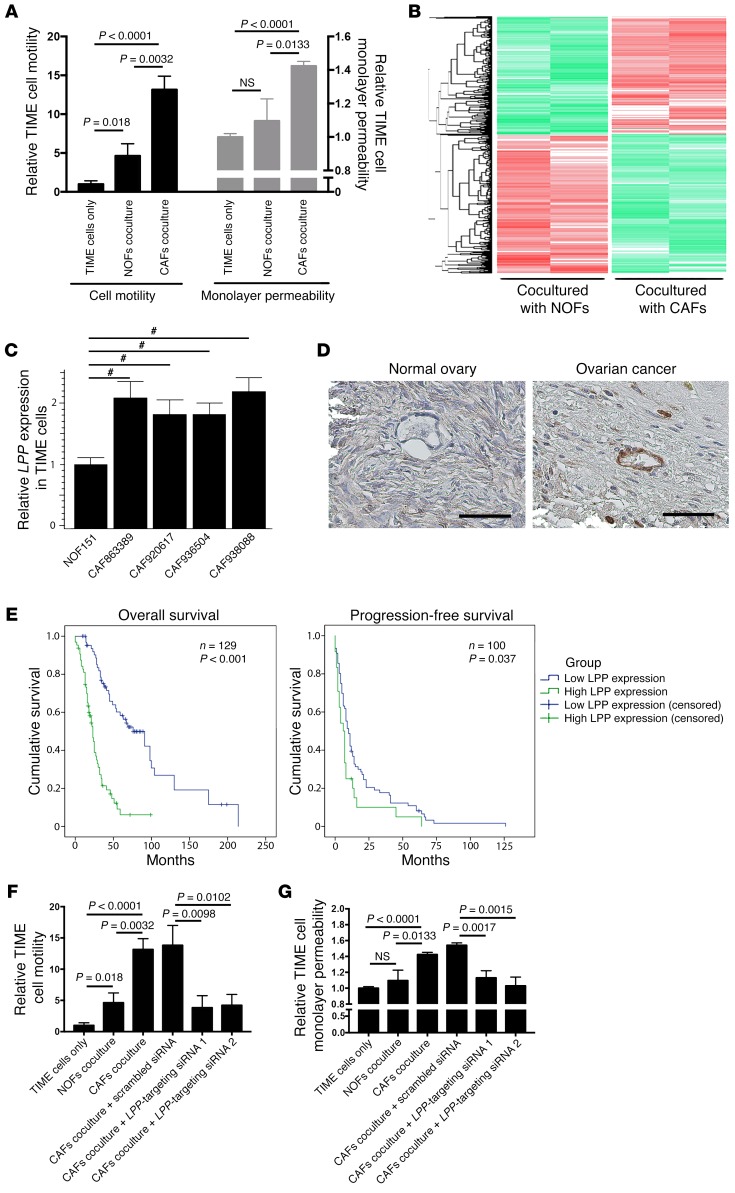
Figure 1. CAF-induced endothelial LPP expression in ovarian cancer.
(A) TIME MECs cocultured with CAFs had significantly higher motility rates and monolayer permeability compared with MECs cocultured with NOFs. P values were determined by 2-tailed Student’s t test. (B) Heatmap generated from transcriptome analyses of RNA samples isolated from TIME cells cocultured with CAFsor NOFs. A total of 1,394 genes and 2,106 genes wereup- and downregulated, respectively, in TIME cells cocultured with CAFs versus MECs cocultured with NOFs (fold change >1.5; Benjamini-Hochberg multiple testing–adjusted P < 0.05). LPP was identified as one of the significantly upregulated genes. (C) Quantitative reverse transcription PCR (qRT-PCR) analyses of endothelial cells RNA samples confirmed that endothelial LPP expression was upregulated in the presence of CAFs (#P < 0.0001, by 2-tailed Student’s t test). (D) Hematoxylin- counterstained images of immunolocalization of LPP in a normal ovary and a high-grade serous ovarian cancer showing that ovarian tumor MECs had higher LPP expression levels than did normal ovarian MECs. Scale bars: 50 μm. (E) Kaplan-Meier analysis were used to evaluate the clinical relevance of endothelial LPP expression in patients with HGSC. Elevated endothelial LPP expression was associated with lower overall and progression-free survival. The median overall survival rate of HGSC patients with high endothelial LPP levels (23 months) was significantly shorter than that of patients with low endothelial LPP levels (76 months) (n = 129; P < 0.001, by log-rank test). The median progression-free survival rate duration of HGSC patients with high endothelial LPP levels (6 months) was significantly shorter than that of patients with low endothelial LPP levels (10 months) (n = 100; P < 0.037, by log-rank test). (F) CAFs increased endothelial cell motility, and the motility-promoting effect of CAFs was attenuated in endothelial cells transfected with LPP-targeting siRNAs. Motility assays were performed using Boyden chambers. Endothelial cells in the upper chamber were allowed to migrate through the porous membrane in the presence of CAFs or NOFs in the bottom chamber (P values were determined by 2-tailed Student’s t test). (G) CAFs increased the permeability of a confluent endothelial cell monolayer, and the permeability-enhancing effect of CAFs was attenuated in endothelial cells transfected with LPP-targeting siRNAs (P values were determined by 2-tailed Student’s t test). Fluorescence-labeled dextran was added to a confluent monolayer culture of endothelial cells in the upper chamber of a Boyden chamber and the amount of dextran diffusing through the endothelial cell monolayer culture in the presence of CAFs or NOFs to the lower chamber was measured by an ELISA microplate reader. All data represent the mean ± SEM of 3 independent experiments.