Figure 2.
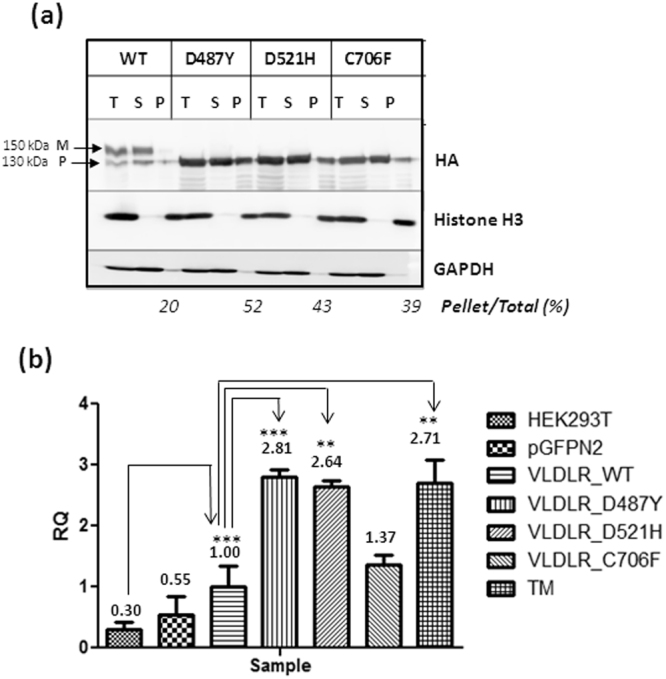
Analysis of aggregation states of VLDLR mutants and measurement. of ER stress (a) Analysis of VLDLR solubility in the nonionic detergent Triton X-100. HEK-293T cells were transiently transfected with the indicated plasmids. Cell extracts were prepared in lysis buffer supplemented with 1% Triton X-100 and centrifuged at 4 °C at 20,000 × g for 15 min. The total cell lysate (T), pellet (P), and supernatant (S) fractions were analyzed for presence of respective VLDLR proteins by western blot against HA. The mature (M) and precursor (P) forms of VLDLR are indicated by arrows Histone H3 and GAPDH were used as controls for pellet and soluble fractions respectively. The experiment was performed twice with similar results. Regions cropped from separate images are demarcated with borders. Unprocessed original scans of blots are shown in Supplementary Figure S9. (b) Induction of ER stress in HEK-293T cells 48 h post transfection with VLDLR WT or mutants. Aggregation-prone VLDLR mutants induce ER stress, represented through elevated alternatively spliced XBP-1 transcript levels, measured through quantitative PCR (qPCR). The XBP-1s mRNA levels of the VLDLR WT at 48 h post-transfection was set as 1.00. Fold-changes mRNA expression of the mutants were expressed in relation to WT. Error bars represent ± S.E.M. of three experiments; (*) p ≤ 0.05; (**) p ≤ 0.01; (***) p ≤ 0.001; One-way ANOVA, Bonferroni post-test.
