Abstract
The factors that lead to the improvement of gait function in patients with diseases of the central nervous system (CNS) who use a hybrid assistive limb (HAL) are not yet fully understood. The purpose of the present study was to analyze these factors to determine the prognosis of the patients’ gait function. Patients whose CNS disease was within 180 days since onset were designated as the subacute-phase patients, and patients whose disease onset had occurred more than 180 days previously were designated as chronic-phase patients. Fifteen subacute-phase patients and 15 chronic-phase patients were given HAL training. The study analyzed how post-training walking independence in these patients was affected by the following factors: age, disease, lesion area, lower limb function, balance, period until the start of training, number of training sessions, additional rehabilitation, higher-order cognitive dysfunction, HAL model, and the use of a non-weight-bearing walking-aid. In subacute-phase patients, walking independence was related to lower limb function (rs = 0.35). In chronic-phase patients, there was a statistically significant correlation between post-training walking independence and balance (rs = 0.78). In addition, in patients with a severe motor dysfunction that was accompanied by inattention and global cognitive dysfunction, little improvement occurred, even with double-leg model training, because they had difficulty wearing the device. The results demonstrated that the factors that improved walking independence post HAL training differed between patients with subacute- and chronic-stage CNS diseases. The findings may serve as valuable information for future HAL training of patients with CNS diseases.
Keywords: gait function, hybrid assistive limb, neurorehabilitation, central nervous system disease
Introduction
Walking is an important ability for everyday living, and for humans, it provides a high degree of freedom to respond to changes in the environment. Most patients with central nervous system (CNS) diseases experience motor paralysis of the lower limbs, and their gait function is impaired, which restricts movement in daily life.
In recent years, various gait training assistive robots have been developed, such as the Rehabot,1) Gait Trainer,2,3) Lokomat,4) and LOPES Exoskeleton Robot,5) and rehabilitation using robots is becoming more widespread. The hybrid assistive limb ([HAL], CYBERDYNE, Inc., Tsukuba, Japan) is a cyborg-type gait-assistive robot that was developed by Sankai et al.6,7) to assist with walking. It features a voluntary control function that complies with the wishes of the wearer, a function that was developed through the fusion of medicine and engineering. HAL is a new type of neurorehabilitation tool that supports the automatic movement of the hip and knee joints based on the bioelectrical signals (BES) from the wearer’s rectus femoris, vastus lateralis, biceps femoris, and gluteus maximus. Simultaneously, this suit uses information on the position of the center of gravity to determine the stance or swing phases, possesses functions that detect voluntary human movement, and offers a technological, automatic control capability to support the movement of the lower limbs in synchronization with the gait cycle.
Gait training with the gait-assistive robot suit HAL has been reported to improve the walking ability of patients with either acute8–10) or chronic stroke,11) or spinal cord injury,12,13) and effectively reduces the amount of assistance during the wearer’s activities of daily livings (ADLs).14) However, there is little information on the application of HAL to rehabilitate patients with gait disorders and CNS diseases such as stroke, brain tumor, and spinal cord injury. Therefore, uncovering the factors affecting the prognosis of patient function, particularly patient characteristics such as age, disease, disease site, cognitive dysfunction, lower limb function, balance, and intervention period, will likely provide valuable information when considering how to apply HAL training.
The purpose of this study was to analyze the factors affecting the prognosis of gait function after HAL training in patients with CNS diseases.
Materials and Methods
The gait disturbance was due to intracerebral hemorrhage in 15 patients, cerebral infarction in 4, meningioma in 5, diffuse axonal injury in 3, and spinal cord disease in 3, with a consciousness level of grade 1 or grade 0 on the Japan Coma Scale (JCS).15) The study was approved by the ethical committee of the University of the Ryukyus (No. 377), and training using HAL was provided to 30 patients who were given a written explanation about their cooperation in the study and from whom informed consent to participate was obtained. We classified patients who had started HAL training within 180 days since the onset of disease into the subacute-phase group, and those who started HAL training after at least 180 days since disease onset were classified into the chronic-phase group.16,17) The subacute group (cases 1–15) consisted of 10 women and 5 men with a median age of 61 years (range: 18 to 86 years). All patients in the subacute-phase group had a lower limb deficit and gait disorder. The chronic group (cases 16–30) consisted of 3 women and 12 men with a median age of 59 years (range: 19 to 83 years). All patients in the chronic-phase group had a lower limb deficit, and 11 patients in this group had a gait disorder. The details of each patient’s profile are given in Table 1.
Table 1.
Clinical features of the patients in this study
| Case | Age | Sex | Diagnosis | Location | Type of paralysis | Higher-order cognitive dysfunction | Period from the onset (days) | Type of HAL |
|---|---|---|---|---|---|---|---|---|
| Case 1 | 80 | M | olfactory groove meningioma | frontal | paraplegia | global cognitive function | 25 | double |
| Case 2 | 86 | F | falx meningioma | parietal | hemiplegia | executive function | 8 | single |
| Case 3 | 61 | F | parasagittal meningioma | frontal | hemiplegia | executive function, phycomotor speed | 26 | single |
| Case 4 | 57 | F | ICH | frontal | hemiplegia | - | 16 | double |
| Case 5 | 69 | F | ICH | putamen | hemiplegia | transcortical motor aphasia | 8 | double |
| Case 6 | 55 | M | ICH | thalamus | hemiplegia | - | 4 | single |
| Case 7 | 62 | F | ICH | parietal | hemiplegia | - | 5 | single |
| Case 8 | 38 | F | ICH | putamen | hemiplegia | transcortical motor aphasia | 8 | single |
| Case 9 | 51 | F | SAH | insular | hemiplegia | executive function, pusher behavior | 21 | double |
| Case 10 | 72 | F | cerebral infarction, schizophrenia | frontal, parietal, putamen, temporal, insula | hemiplegia | unilateral spatial neglect | 22 | double |
| Case 11 | 76 | M | cerebral infarction | cerebral peduncle, cerebellum | hemiplegia | - | 15 | single |
| Case 12 | 18 | M | DAI | orbitofrontal, parietal, temporal pole | hemiplegia | phycomotor speed, working memory | 79 | single |
| Case 13 | 74 | F | petroclival meningioma, cerebral infarction | brain stem | hemiplegia | - | 20 | single |
| Case 14 | 52 | M | cerebral infarction | corona radiate | hemiplegia | - | 16 | single |
| Case 15 | 61 | F | ICH | parietal | hemiplegia | constructional apraxia | 21 | single |
| Case 16 | 83 | M | parasagittal meningioma | frontal | hemiplegia | - | 13149 | single |
| Case 17 | 53 | F | ICH | putamen | hemiplegia | - | 801 | single |
| Case 18 | 60 | M | ICH | putamen | hemiplegia | transcortical motor aphasia | 237 | single |
| Case 19 | 61 | M | ICH | thalamus | hemiplegia | - | 858 | single |
| Case 20 | 60 | F | ICH | parietal | hemiplegia | motor aphasia | 592 | single |
| Case 21 | 40 | M | ICH | insula | hemiplegia | motor aphasia | 5844 | single |
| Case 22 | 57 | M | ICH | putamen | hemiplegia | transcortical motor aphasia | 763 | single |
| Case 23 | 64 | F | ICH | frontal | hemiplegia | motor aphasia | 609 | single |
| Case 24 | 75 | M | ICH | thalamus | hemiplegia | executive function, phycomotor speed | 2708 | single |
| Case 25 | 59 | M | cerebral infarction | frontal, parietal, putamen, insula | hemiplegia | transcortical motor aphasia | 4078 | single |
| Case 26 | 22 | M | DAI | corpus callosum, corona radiata (R), temporal (L), occipital (L) | quadriplegia | global cognitive function | 413 | double |
| Case 27 | 19 | M | DAI | corpus callosum, corona radiata, cerebellar vermis | hemiplegia | working memory | 586 | single |
| Case 28 | 69 | M | spinal cord injury | C5, C6 | quadriplegia | executive function, working memory | 2464 | double |
| Case 29 | 50 | M | syringomyelia | C1∼T5 | quadriplegia | - | 2246 | double |
| Case 30 | 44 | M | dural AVF | T6∼T8 | hemiplegia | - | 1403 | single |
AVF: arteriovenous fistula, C: cervical nerves, DAI: diffuse axonal injury, double: double-leg model with exoskeleton frame, F: female, HAL: hybrid assistive limb, ICH: intracerebral hemorrhage, M: male, single: single-leg model with exoskeleton frame, SAH: subarachnoid hemorrhage, T: thoracic nerves.
HAL training was conducted for 30 minutes once daily. The time required to put on or remove the HAL was not included in the training time. The HAL training program consisted of sitting balance, standing balance, and gait training. Training was conducted with patients wearing either a single-leg or double-leg HAL model. Patients with paraplegia or quadriplegia and/or patients who could not maintain a sitting position used the double-leg HAL model. The single-leg HAL model was applied to patients with hemiplegia who could maintain a sitting position.18) Patients who could not maintain a sitting or standing position used an All-in-One Walking Trainer (All-in-One, manufactured by ROPOX A/S, Næstved, Denmark). A dedicated sling (DOMINO Slings, manufactured by ROPOX A/S) for the All-in-One was used, and because it could support the pelvis of the wearer and hold up the trunk of the body, it even allowed patients who were unable to maintain a seated or standing position to do so safely.
Gait function, lower limb function, balance, and ADLs were each evaluated according to the Functional Ambulation Categories (FAC),19) Fugl-Meyer Assessment (FMA),20) and Functional Independence Measure (FIM)21,22) before HAL training started and when it was finished.
The neuropsychological assessments that were used are shown below. The Mini-Mental State Examination (MMSE)23) and a modified MMSE (3MS)24) were used for the global cognitive screening assessment. The flexibility of the executive function of the frontal lobe was evaluated with the Trail Making Test (TMT),25) and cognitive inhibition was evaluated with the Stroop Test (ST).25) The Wechsler Adult Intelligence Scale-Revised (WAIS-R) digit span subtest (DS),26) which reveals the retention operation of working memory, was used to evaluate memory function. Furthermore, the WAIS-R digit symbol test (DST)26) was used to evaluate the psychomotor speed of the entire brain. To evaluate the visuospatial ability of the parietal lobe, we used a partial WAIS-R block design subtest26) and the cube-copying test.
Spearman’s rank-order correlation analysis was used to calculate correlations between the FAC when HAL training was finished and age, the period from disease onset until the start of training, number of training sessions, and scores of lower limb function and balance before training. The range of the rank-order correlation coefficient (rs) was between −1 and +1. When the rs value was close to +1, it indicated that there was a positive correlation between the FAC and scores of the other functional assessment items. Conversely, when the rs value was close to −1, there was a negative correlation between the FAC and other functional assessment items. The strength of the rs value was assessed using five categories: negligible correlation (0.00 to 0.30 or 0.00 to −0.30), low correlation (0.30 to 0.50 or −0.30 to −0.50), moderate correlation (0.50 to 0.70 or −0.50 to −0.70), high correlation (0.70 to 0.90 or −0.70 to −0.90), and very high correlation (0.90 to 1.00 or −0.90 to −1.00).27,28) If the strength of the rs value was more than 0.3, it was considered to be positively correlated, and if it was less than −0.3, it was considered to be negatively correlated. Fisher’s exact test was used to test the significance between the number of patients who were capable (FAC > 1) and incapable of walking (FAC = 0) when HAL training was finished, according to seven factors: (1) lesion side, (2) disease type, (3) lesion site, (4) presence or absence of higher-order cognitive dysfunction, (5) HAL specification, (6) additional use of the All-in-One, and (7) additional occupational therapy (OT) or physical therapy (PT) (significance level: 5%). In addition, the Wilcoxon rank-sum test was used to assess the difference in the median values of FAC, FMA (lower limb function and balance), and FIM before HAL training started and when it was finished (significance level: 5%).
Results
HAL training was provided to 15 patients with subacute-phase CNS disease and 15 patients with chronic-phase disease. The details of each group are given in Table 2.
Table 2.
Summary of patients’ characteristics
| Characteristics | Subacute phases | Chronic phases | ||
|---|---|---|---|---|
| n = 15 | % | n = 15 | % | |
| Higher-order cognitive dysfunction | ||||
| yes | 8 | 53% | 8 | 53% |
| no | 7 | 47% | 7 | 47% |
| Period from the onset to starting training (range) | 13 (5–79) | 858 (237–13149) | ||
| Number of training sessions (median) (range) | 5 (5–15) | 5 (5–10) | ||
| Specification of HAL | ||||
| single-leg model | 10 | 67% | 12 | 80% |
| double-leg model | 5 | 33% | 3 | 20% |
| Application of All-in-one | ||||
| yes | 10 | 67% | 6 | 38% |
| no | 5 | 33% | 9 | 56% |
| Application of OT or PT | ||||
| yes | 15 | 100% | 1 | 7% |
| no | 0 | 0% | 14 | 93% |
| FAC before HAL training | ||||
| FAC ≥ 1 | 0 | 0% | 12 | 80% |
| FAC = 0 | 15 | 100% | 3 | 20% |
FAC: functional ambulation classification, HAL: hybrid assistive limb, OT: occupational therapy, PT: physical therapy. The integer denotes the number of patients.
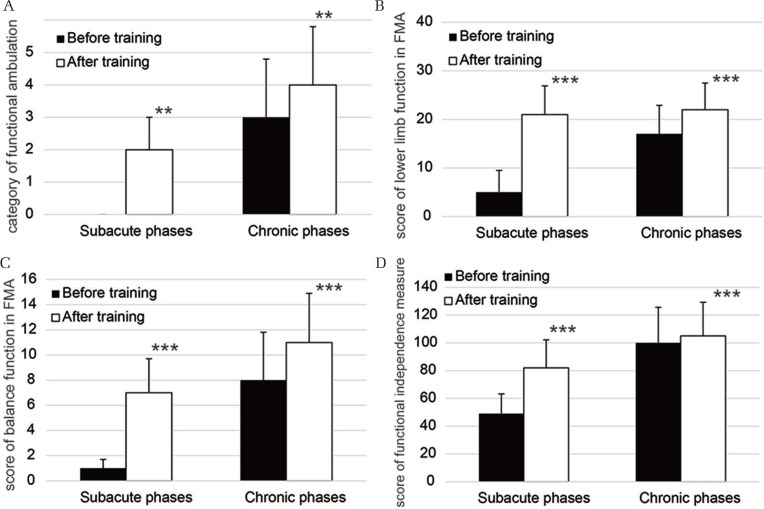
All functional assessments in the patients with subacute-phase disease and the those with chronic-phase disease showed that there was improvement. The patients whose disease was in the subacute phase demonstrated a significant increase (P < 0.01) in the median FAC score from 0 ± 0 (range: 0–0) before training started to 2 ± 1 (range: 0–4) when training was finished (Fig. 1A). The walking categories in the chronic phase group significantly increased (P < 0.01) from baseline (median: 3 ± 1; range: 0–5) to after training (median: 4 ± 1.8; range: 0–5) (Fig. 1A). The median score for lower limb function in the subacute and chronic-phase groups significantly improved from before training (5 ± 4.5; range: 1–14) to after training (21 ± 5.9; range: 9–29, P < 0.001), and from 17 ± 5.9 (range: 4–25) to 22 ± 5.5 (range: 9–27, P < 0.001), respectively (Fig. 1B). The balance scores of the FMA in the patients whose disease was in the subacute phase significantly increased (P < 0.001) from baseline (median: 1 ± 0.7; range: 0–2) to when training was finished (median: 7 ± 2.7; range: 2–11) (Fig. 1C). A significant increase (P < 0.001) in the median score for balance from 8 ± 3.8 (range: 0–11) to 11 ± 3.9 (range: 3–13) was observed in the patients whose disease was in the chronic phase (Fig. 1C). The median scores for ADLs in patients in both the subacute and chronic-phase groups showed a significant improvement (P < 0.001) from 49 ± 14.2 (range: 28–70) to 82 ± 20.2 (range: 40–104), and from 100 ± 25.7 (range: 34–120) to 105 ± 24.2 (range: 45–121), respectively (Fig. 1D).
Fig. 1.
Change in functional assessments of the patients in the subacute-phase disease group and chronic-phase disease group. The bar graphs show the median scores for the functional ambulation category before HAL training and after HAL training (A). The bar graphs illustrate the median scores for lower extremity function in the Fugl-Meyer assessment (FMA) (B). The bar graphs demonstrate the median scores for balance in the FMA (C). The bar graphs show the median scores of functional independent measure (D). ** and *** denote P < 0.01 and P < 0.001, respectively (the Wilcoxon rank-sum test).
In the subacute-phase group, Fisher’s exact test revealed that there was no significant bias in the number of patients who were capable (FAC ≥ 1) and incapable of walking (FAC = 0) after training (Table 3). In the chronic-phase group, Fisher’s exact test showed that there was a significant bias in the number of patients who were capable and incapable of walking after training, according to the affected side (P < 0.05) and the HAL specification that was used for training (P < 0.05) (Table 3). These results showed that bilateral-side lesions and training with the double-leg HAL model affected gait function after HAL training in patients with chronic-phase disease.
Table 3.
Clinical factors influenced on gait function after HAL training finished
| Factor | Subacute phases | P | Chronic phases | P | ||
|---|---|---|---|---|---|---|
| FAC = 0 | FAC ≥ 1 | FAC = 0 | FAC ≥ 1 | |||
| Side of lesion | 0.64 | 0.03 | ||||
| Left | 1 | 4 | 0 | 7 | ||
| Rgiht | 1 | 8 | 0 | 5 | ||
| Bilateral | 0 | 1 | 2* | 1 | ||
| Diagnosis | 0.61 | 0.2 | ||||
| intracerebral hemorrhage | 1 | 6 | 0 | 8 | ||
| cerebral infarction | 1 | 2 | 0 | 1 | ||
| meningioma | 0 | 4 | 0 | 1 | ||
| diffuse axonal injury | 0 | 1 | 1 | 1 | ||
| spinal cord diseases | 0 | 0 | 1 | 2 | ||
| Location of lesion | 0.88 | 0.64 | ||||
| frontal lobe | 1 | 4 | 0 | 4 | ||
| parietal lobe | 1 | 4 | 0 | 2 | ||
| temporal lobe | 1 | 1 | 0 | 0 | ||
| insula cortex | 1 | 1 | 0 | 2 | ||
| putamen | 1 | 2 | 0 | 4 | ||
| thalamus | 0 | 0 | 0 | 2 | ||
| corpus callosum | 0 | 0 | 1 | 1 | ||
| corona radiata | 1 | 1 | 1 | 2 | ||
| brainstem | 0 | 2 | 0 | 1 | ||
| cerebellum | 0 | 1 | 0 | 1 | ||
| spinal cord | 0 | 0 | 1 | 2 | ||
| Higher-order cognitive dysfunction | 0.28 | 0.5 | ||||
| yes | 2 | 8 | 2 | 8 | ||
| no | 0 | 5 | 0 | 5 | ||
| Specification of HAL | 0.09 | 0.03 | ||||
| single-leg type | 0 | 10 | 0 | 12* | ||
| double-leg type | 2 | 3 | 2* | 1 | ||
| Application of All-in-one | 0.09 | 0.18 | ||||
| yes | 0 | 10 | 2 | 4 | ||
| no | 2 | 3 | 0 | 9 | ||
| Combination of OT or PT | 1.0 | 1.0 | ||||
| yes | 2 | 13 | 0 | 1 | ||
| no | 0 | 0 | 2 | 12 | ||
FAC: functional ambulation classification, FAC = 0: number of patients who cannot ambulate, FAC ≥ 1: number of patients are classified as from 1 to 5 in FAC, HAL: hybrid assistive limb, OT: occupational therapy, PT: physical therapy, P: P-value of Fisher’s exact test,
P < 0.05 (residual analysis).
The results of the Spearman’s rank-order correlation analysis and FMA scores for lower limb function showed that there was a low positive correlation (rs = 0.35, P = 0.09) with the FAC scores after HAL training in the subacute-phase patients (Table 4). The chronic-phase diseases demonstrated a high positive correlation (rs = 0.78, P = 0.003) with the FMA scores for balance, and a low positive correlation (rs = 0.36, P = 0.09) with the scores for lower extremity function (Table 4).
Table 4.
Spearman’s rank-order correlation coefficients of gait function and age, period from the onset, number of training sessions, function of lower extermity and postural control before HAL training, respectively
| Variables | Subacute phases | Chronic phases |
|---|---|---|
| Age | −0.19 | −0.18 |
| Period from the onset | −0.14 | 0.24 |
| Number of training sessions | 0.06 | −0.28 |
| Lower limb score of FMA before training | 0.35 | 0.36 |
| Balance score of FMA before training | 0.24 | 0.78*** |
FMA: Fugl-Meyer assessment, HAL: hybrid-assistive limb,
P < 0.001.
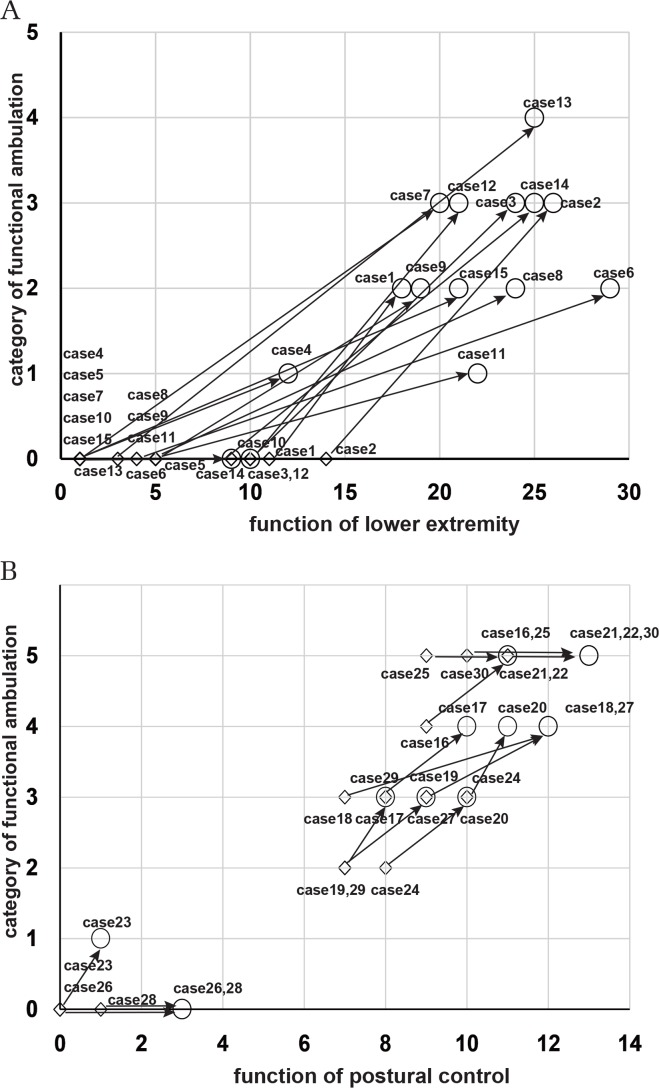
The walking categories and the lower limb function scores for individual patients whose disease was in the subacute phase are shown in Fig. 2A. The arrows show the change in gait and lower limb function from before training started to when training was finished. The patients with a lower limb function score of 8 or higher before training experienced an improvement of the FAC score to 3 after HAL training. The patients with a lower limb function score of 5 or lower before training tended to have an FAC score that improved to 2. Figure 2B shows the change in gait function and balance scores of patients in the chronic-phase group from the start of training until it was finished. The FAC of 9 patients increased by one level after HAL training was finished, and the balance scores also improved. Eight patients who had a balance score of 7 or higher before training improved to an FAC of 3 or higher when training was finished, and 5 patients, in particular, regained walking independence. After training, there was an increase in the balance scores, but there was no change in the FAC of 2 patients with an FAC of 0 and 4 patients with an FAC of 5. Four of the 15 patients who used the double-leg model (patients 5, 10, 26, and 28) remained unable to walk. Patients 5, 10, 26, and 28 had severe motor dysfunction or quadriplegia that was complicated by higher-order cognitive dysfunction.
Fig. 2.
Alteration of the walking category and physical function in the patients in the subacute-phase disease group and chronic-phase disease group. A scatterplot illustrates the categories of functional ambulation (y axis) and scores of lower limb function (x axis) before and after HAL training in each patient with a subacute CNS disease (A). The square and circle denote data from pre-training and post-training, respectively. The arrow represents alteration of walking ability and lower limb function in patients after HAL training. A scatterplot graph illustrates the categories of functional ambulation (y axis) and scores for postural control (x axis) before and after HAL training in each patient with a chronic CNS disease (B). The arrows represent improvement in gait function and postural control in patients after HAL training.
Discussion
The goal of this study was to analyze the factors that are related to the prognosis of gait after HAL training in patients with lower limb motor paralysis due to CNS diseases such as stroke and benign brain tumor. We analyzed the factors that affect the prognosis of gait function after HAL training in patients with subacute- and chronic-phase disease.
The traditional rehabilitation approach, neurodevelopmental treatment, and/or robotic-assisted locomotor training for patients in the acute and chronic phases of CNS disease improved their dysfunction of the lower limb and standing balance, as well as walking ability.4,8–11,17,29,30–32) The robot-assisted training that included HAL restored the gait function and/or ADLs of patients after the subacute stage of stroke, compared with conventional rehabilitation.4,14,16,33) There is not much difference in the walking ability and/or balance of patients in the chronic stage after stroke between rehabilitation with Locomat and the conventional rehabilitation.31,34) Recently, several reports indicated that gait training with robot-suit HAL improved gait function and standing balance in patients in the chronic phase after stroke.11,34,35) Our study demonstrated that HAL training improved not only the walking ability but also the function of the lower limb, balance, and ADLs of patients with CNS diseases, both in the subacute and chronic phases. Indeed, in our study, HAL training effectively improved the function of patients with the chronic stage of CNS disease, although we found that it was difficult to recover the patients’ motor functioning using conventional rehabilitation.36)
Lower limb function, represented by an FMA score of 4 or above, indicated that the tendon reflex and synergistic pattern in voluntary movement were normal.20) The BES-induced, synergistic movement of the lower limbs is triggered using HAL gait assistance.6,7) Although, the effect of HAL on gait function in patients with mild motor paralysis has been reported,8) in this study, we found that starting HAL training soon after CNS disease onset or an operation led to effective improvement of gait disorders in patients with severe motor paralysis.
The double-leg HAL model is an effective tool for the gait training of patients with paraplegia or quadriplegia with a spinal cord injury and/or severe brain injury.10,18,34) Patients with global cognitive dysfunction and inattention remained unable to walk in this study, and could not be expected to improve their walking ability.37,38) For the double-leg HAL model to work, the wearer must use knee or hip joint movements such as bending and elongation in both legs; therefore, compared with the single-leg type model, attention must be paid to more joints, and more movement control is required.6,7)
The stability of standing balance is closely associated with the stance phase of a bipedal gait cycle.39–43) In each step, the patient must be able to adjust the posture of the head and body so that the body’s center of gravity (CG) does not fly out from the base of support.40,41,44) HAL supports the movement of the lower limbs,6,7) but the postural adjustment of the head and body depends mostly on the ability of the wearer. Patients with good standing balance may experience improvements in their walking ability because the HAL training facilitates forward propulsion of the CG and alignment of the lower limb during the stance phase.6,7,45)
This study had several limitations. Firstly, this was a retrospective study, and it was not randomized or double-blinded. Next, the number of patients with subacute- or chronic-stage disease was very small. We plan to perform a randomized double-blind study with a larger number of subjects to have solid proof of the utility of HAL for training. The factors that help improve the effect of HAL training on lower limb function and balance must be further analyzed.
In conclusion, we found that the walking ability of patients with CNS disease after HAL training was influenced by lower limb function, standing balance, and higher-order cognitive dysfunction. These findings are valuable for predicting the prognosis of gait function after HAL training in patients with a CNS disease and for considering the application of HAL to rehabilitation.
Acknowledgement
This study was partially supported by Industrial Disease Clinical Research Grants from the Ministry of Health, Labour, and Welfare (to S.I., Y.H., and M.N.) and a Grant-in-Aid for Scientific Research (C) (15K01465) (to M.N.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
Conflicts of Interest Disclosure
None declared. All authors who are members of The Japan Neurosurgical Society (JNS) have registered self-reported COI disclosure statement forms through the website for JNS members.
References
- 1).Siddiqi NA, Ide T, Chen MY, Akamatsu N: A computer-aided walking rehabilitation robot. Am J Phys Med Rehabil 73: 212–216, 1994 [DOI] [PubMed] [Google Scholar]
- 2).Pohl M, Werner C, Holzgraefe M, et al. : Repetitive locomotor training and physiotherapy improve walking and basic activities of daily living after stroke: a single-blind, randomized multicentre trial (DEutsche GAngtrainerStudie, DEGAS). Clin Rehabil 21: 17–27, 2007 [DOI] [PubMed] [Google Scholar]
- 3).Hesse S, Werner C, Pohl M, Rueckriem S, Mehrholz J, Lingnau ML: Computerized arm training improves the motor control of the severely affected arm after stroke: a single-blinded randomized trial in two centers. Stroke 36: 1960–1966, 2005 [DOI] [PubMed] [Google Scholar]
- 4).Schwartz I, Sajin A, Fisher I, et al. : The effectiveness of locomotor therapy using robotic-assisted gait training in subacute stroke patients: a randomized controlled trial. PM R 1: 516–523, 2009 [DOI] [PubMed] [Google Scholar]
- 5).Veneman JF, Kruidhof R, Hekman EE, Ekkelenkamp R, Van Asseldonk EH, van der Kooij H: Design and evaluation of the LOPES exoskeleton robot for interactive gait rehabilitation. IEEE Trans Neural Syst Rehabil Eng 15: 379–386, 2007 [DOI] [PubMed] [Google Scholar]
- 6).Kawamoto H, Sankai Y: Power assist method based on phase sequence and muscle force condition for HAL. Advanced Robotics 19: 717–734, 2005 [Google Scholar]
- 7).Lee S, Sankai Y: Virtual impedance adjustment in unconstrained motion for an exoskeletal robot assisting the lower limb. Advanced Robotics 19: 773–795, 2005 [Google Scholar]
- 8).Fukuda H, Samura K, Hamada O, et al. : Effectiveness of acute phase hybrid assistive limb rehabilitation in stroke patients classified by paralysis severity. Neurol Med Chir (Tokyo) 55: 487–492, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Nilsson A, Vreede KS, Häglund V, Kawamoto H, Sankai Y, Borg J: Gait training early after stroke with a new exoskeleton—the hybrid assistive limb: a study of safety and feasibility. J Neuroeng Rehabil 11: 92, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Ueba T, Hamada O, Ogata T, Inoue T, Shiota E, Sankai Y: Feasibility and safety of acute phase rehabilitation after stroke using the hybrid assistive limb robot suit. Neurol Med Chir (Tokyo) 53: 287–290, 2013 [DOI] [PubMed] [Google Scholar]
- 11).Kawamoto H, Kamibayashi K, Nakata Y, et al. : Pilot study of locomotion improvement using hybrid assistive limb in chronic stroke patients. BMC Neurol 13: 141, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Sczesny-Kaiser M, Höffken O, Aach M, et al. : HAL® exoskeleton training improves walking parameters and normalizes cortical excitability in primary somatosensory cortex in spinal cord injury patients. J Neuroeng Rehabil 12: 68, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Wall A, Borg J, Palmcrantz S: Clinical application of the hybrid assistive limb (HAL) for gait training: a systematic review. Front Sys Neurosci 9: 48, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Ogata T, Abe H, Samura K, et al. : Hybrid assistive limb (HAL) rehabilitation in patients with acute hemorrhagic stroke. Neurol Med Chir (Tokyo) 55: 901–906, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Yagi T, Saito N, Hara Y, Matumoto HH, Mashiko K: Japan coma scale used in the prehospital setting can predict clinical outcome in severe pediatric trauma. Critical Care 17: P324, 2013 [Google Scholar]
- 16).Ng MF, Tong RK, Li LS: A pilot study of randomized clinical controlled trial of gait training in subacute stroke patients with partial body-weight support electromechanical gait trainer and functional electrical stimulation: six-month follow-up. Stroke 39: 154–160, 2008 [DOI] [PubMed] [Google Scholar]
- 17).Werner C, Von Frankenberg S, Treig T, Konrad M, Hesse S: Treadmill training with partial body weight support and an electromechanical gait trainer for restoration of gait in subacute stroke patients: a randomized crossover study. Stroke 33: 2895–2901, 2002 [DOI] [PubMed] [Google Scholar]
- 18).Fukuda H, Morishita T, Ogata T, et al. : Tailor-made rehabilitation approach using multiple types of hybrid assistive limb robots for acute stroke patients: a pilot study. Assist Technol 28: 53–56, 2016 [DOI] [PubMed] [Google Scholar]
- 19).Holden MK, Gill KM, Magliozzi MR, Nathan J, Piehl-Baker L: Clinical gait assessment in the neurologically impaired. Reliability and meaningfulness. Phys Ther 64: 35–40, 1984 [DOI] [PubMed] [Google Scholar]
- 20).Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S: The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med 7: 13–31, 1975 [PubMed] [Google Scholar]
- 21).Keith RA, Granger CV, Hamilton BB, Sherwin FS: The functional independence measure: a new tool for rehabilitation. Adv Clin Rehabil 1: 6–18, 1987 [PubMed] [Google Scholar]
- 22).Linacre JM, Heinemann AW, Wright BD, Granger CV, Hamilton BB: The structure and stability of the Functional Independence Measure. Arch Phys Med Rehabil 75: 127–132, 1994 [PubMed] [Google Scholar]
- 23).Folstein MF, Folstein SE, McHugh PR: “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189–198, 1975 [DOI] [PubMed] [Google Scholar]
- 24).Teng EL, Chui HC: The modified mini-mental state (3MS) examination. J Clin Psychiatry 48: 314–318, 1987 [PubMed] [Google Scholar]
- 25).Lezak M, Howieson DB, Loring DW: Orientation and attention. In Muriel Lezak MD, Howieson DB, Bigler ED, Tranel D. (eds): Neuropsychological assessment 4th ed New York, Oxford University Press, 2004, pp. 365–368, 371–364 [Google Scholar]
- 26).Kaufman AS: Test Review: Wechsler, D. Manual for the Wechsler Adult Intelligence Scale, Revised. New York: Psychological Corporation, 1981. J Psychoeducational Assessment 1: 309–313, 1983 [Google Scholar]
- 27).Shiroma A, Nishimura M, Nagamine H, et al. : Cerebellar contribution to pattern separation of human hippocampal memory circuits. Cerebellum 15: 645–662, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Mukaka MM: Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J 24: 69–71, 2012 [PMC free article] [PubMed] [Google Scholar]
- 29).Huitema RB, Hof AL, Mulder T, Brouwer WH, Dekker R, Postema K: Functional recovery of gait and joint kinematics after right hemispheric stroke. Arch Phys Med Rehabil 85: 1982–1988, 2004 [DOI] [PubMed] [Google Scholar]
- 30).Hollands KL, Pelton TA, Tyson SF, Hollands MA, van Vliet PM: Interventions for coordination of walking following stroke: systematic review. Gait Posture 35: 349–359, 2012 [DOI] [PubMed] [Google Scholar]
- 31).Bae YH, Ko YJ, Chang WH, et al. : Effects of robot-assisted gait training combined with functional electrical stimulation on recovery of locomotor mobility in chronic stroke patients: a randomized controlled trial. J Phys Ther Sci 26: 1949–1953, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Watanabe H, Tanaka N, Inuta T, Saitou H, Yanagi H: Locomotion improvement using a hybrid assistive limb in recovery phase stroke patients: a randomized controlled pilot study. Arch Phys Med Rehabil 95: 2006–2012, 2014 [DOI] [PubMed] [Google Scholar]
- 33).Yoshikawa K, Mizukami M, Kawamoto H, et al. : Gait training with hybrid assistive limb enhances the gait functions in subacute stroke patients: a pilot study. Neuro Rehabilitation 40: 87–97, 2017 [DOI] [PubMed] [Google Scholar]
- 34).Hornby TG, Campbell DD, Kahn JH, Demott T, Moore JL, Roth HR: Enhanced gait-related improvements after therapist- versus robotic-assisted locomotor training in subjects with chronic stroke: a randomized controlled study. Stroke 39: 1786–1792, 2008 [DOI] [PubMed] [Google Scholar]
- 35).Yoshimoto T, Shimizu I, Hiroi Y, Kawaki M, Sato D, Nagasawa M: Feasibility and efficacy of high-speed gait training with a voluntary driven exoskeleton robot for gait and balance dysfunction in patients with chronic stroke: nonrandomized pilot study with concurrent control. Int J Rehabil Res 38: 338–343, 2015 [DOI] [PubMed] [Google Scholar]
- 36).Hall AL, Bowden MG, Kautz SA, Neptune RR: Biomechanical variables related to walking performance 6-months following post-stroke rehabilitation. Clin Bimech (Bristol, Avon) 27: 1017–1022, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Chihara H, Takagi Y, Nishino K, et al. : Factors predicting the effects of hybrid assistive limb robot suit during the acute phase of central nervous system injury. Neurol Med Chir (Tokyo) 56: 33–37, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Kollen B, van de Port I, Lindeman E, Twisk J, Kwakkel G: Predicting improvement in gait after stroke: a longitudinal prospective study. Stroke 36: 2676–2680, 2005 [DOI] [PubMed] [Google Scholar]
- 39).Hill K, Ellis P, Bernhardt J, Maggs P, Hull S: Balance and mobility outcomes for stroke patients: a comprehensive audit. Aust J Physiother 43: 173–180, 1997 [DOI] [PubMed] [Google Scholar]
- 40).Beauchamp MK, Sibley KM, Lakhani B, et al. : Impairments in systems underlying control of balance in COPD. Chest 141: 1496–1503, 2012 [DOI] [PubMed] [Google Scholar]
- 41).Yang YR, Lee YY, Cheng SJ, Lin PY, Wang RY: Relationships between gait and dynamic balance in early Parkinson’s disease. Gait Posture 27: 611–615, 2008 [DOI] [PubMed] [Google Scholar]
- 42).Shimada H, Obuchi S, Kamide N, Shiba Y, Okamoto M, Kakurai S: Relationship with dynamic balance function during standing and walking. Am J Phys Med Rehabil 82: 511–516, 2003 [DOI] [PubMed] [Google Scholar]
- 43).Murray MP, Seireg AA, Sepic SB: Normal postural stability and steadiness: quantitative assessment. J Bone Joint Surg Am 57: 510–516, 1975 [PubMed] [Google Scholar]
- 44).Hass CJ, Waddell DE, Fleming RP, Juncos JL, Gregor RJ: Gait initiation and dynamic balance control in Parkinson’s disease. Arch Phys Med Rehabil 86: 2172–2176, 2005 [DOI] [PubMed] [Google Scholar]
- 45).Verma R, Arya KN, Sharma P, Garg RK: Understanding gait control in post-stroke: implications for management. J Bodyw Mov Ther 16: 14–21, 2012 [DOI] [PubMed] [Google Scholar]