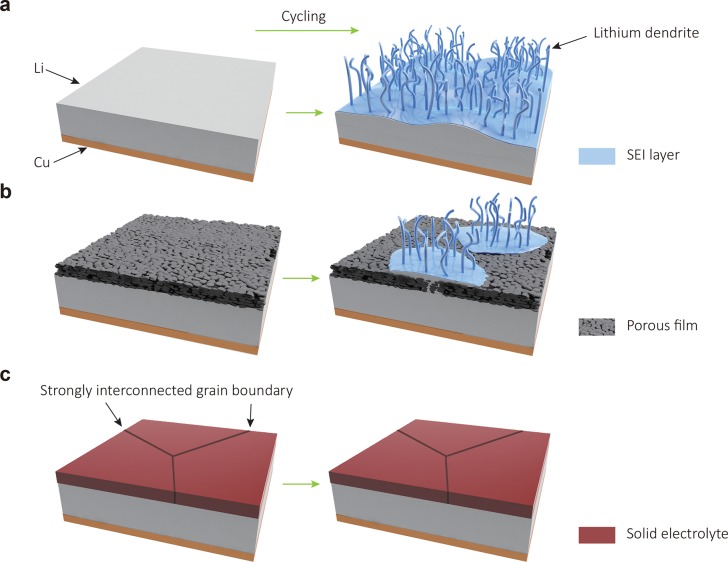
Figure 1.
Design and structure of various Li metal anodes. (a) A bare Li metal anode. Upon cycling, Li dendrites grow and liquid electrolytes are rapidly consumed. (b) A nanofilm modified Li metal anode. The pores and/or cracks of the film can trigger growth of Li dendrites and fail in Li metal surface protection. (c) A Li metal anode with a dense, ionically conductive film with strongly interconnected large grains. A dense protective layer with good mechanical durability, high ionic conductivity, and good adhesion to Li metal anodes can isolate Li metal from corrosive liquid electrolytes and enable stable electrochemical stripping and plating of Li metal underneath, preventing dendrite growth and liquid electrolyte consumption.