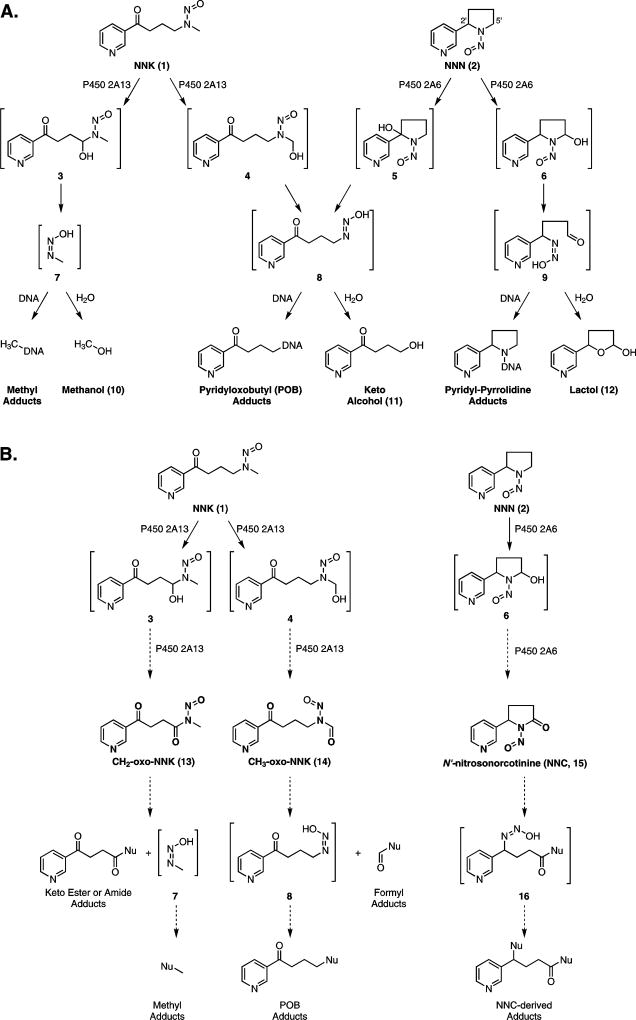
Scheme 1.
(A) Established in vivo metabolism of NNK (1) and NNN (2) by P450 2A13- or P450 2A6-mediated oxidation, respectively. Oxidation results in unstable α-hydroxynitrosamines (3 – 6) which spontaneously decompose to diazohydroxides (7 – 9). These either hydrolyze to products excreted in the urine (10 – 12) or react with DNA to form adducts. (B) Proposed P450-mediated oxidation of NNK (1) and NNN (2) to nitrosamides (13 – 15) through retention of the α-hydroxynitrosamines 3, 4, and 6 within the P450 active site. If formed in vivo, we anticipate these species would also form adducts with DNA.