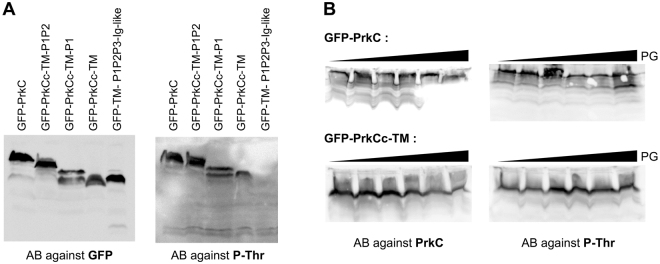
Figure 5.
The kinase activity of PrkC in vivo is not modified by PASTAs deletions or PG binding during exponential growth. (A) PrkC-phosphorylation analysis by western blots. Strains SG278 (ΔprkC, amyE::gfp-prkC), SG467 (ΔprkC, amyE::gfp-prkCc-TM-P1P2), SG466 (ΔprkC, amyE::gfp-prkCc-TM-P1), SG465 (ΔprkC, amyE::gfp-prkCc-TM) and SG497 (ΔprkC, amyE::gfp-TM-P1P2-Ig-like) were grown on LB medium with 0.5% xylose at 37 °C until OD600 = 0.8. After centrifugation, the pellets were resuspended in 1/100th volume of lysis buffer and treated as described in Materials and Methods. For each strain, 16 μl of crude extract were separated by SDS-PAGE. After blotting, phosphorylated PrkC was detected using antibodies directed against P-Thr residues. To estimate the relative quantity of PrkC in crude extracts, we used anti-GFP antibodies. (B) Strains SG278 (ΔprkC, amyE::gfp-prkC) and SG465 (ΔprkC, amyE::gfp-prkCc-TM) were grown on LB medium with 0.5% xylose and increasing amounts of PG fragments (0, 0.003, 0.015, 0.03, 0.3 and 3 µg/ml) at 37 °C until OD600 = 0.8. After centrifugation, the pellets were resuspended in 1/100th volume of lysis buffer and treated as described in Materials and Methods. For each strain, 16 μl of crude extract were separated by SDS-PAGE. After blotting, phosphorylated PrkC was detected using antibodies directed against P-Thr residues. To estimate the relative quantity of PrkC in crude extracts, we used a specific antibody against PrkC. Full-length blots are presented in the supplemental data.