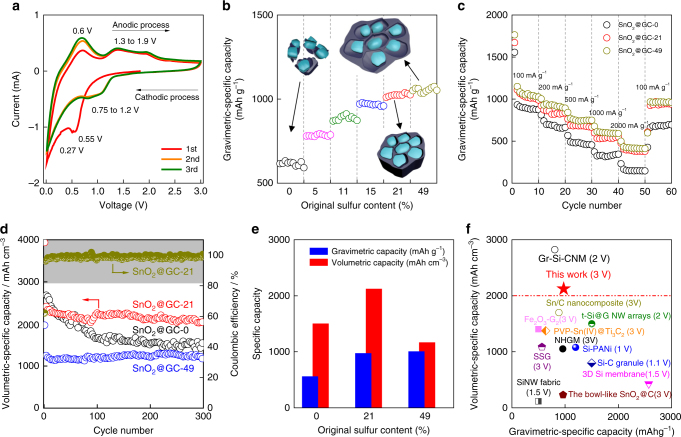
Fig. 4.
Electrochemical characterization of SnO2@GCs. a Representative cyclic voltammetry curves (CVs) of SnO2@GC-21 at a scan rate of 0.5 mV s–1. b The gradient electrochemical performance change of SnO2@GCs based on the different original sulfur contents when the gravimetric capacity became stable at around the 130th–140th cycles (100 mA g–1). The overall cycling performance of SnO2@GCs can be observed in Supplementary Fig. 17. c Rate performance of SnO2@GCs. d Cycling performance of SnO2@GC-0, 21 and 49 at a current density of 100 mA g–1. e, f Comparison of the volumetric and gravimetric specific capacities of SnO2@GC-21 with the referenced cases (based on the total active materials). e Comparison with SnO2@GC-0 and SnO2@GC-49. f Comparison with other reported anode materials (based on the total active materials), such as t-Si@G NW arrays5, Fe2O3-G26, PVP-Sn(IV)@Ti2C7, Sn/C nanocomposite8, SSG9, Si-C granule10, Si-PANi13, NHGM15, SiNW fabric22, 3D Si membrane23 and the bowl-like SnO2@C27, and Gr-Si-CNM45, with a voltage it charged to (see details in Supplementary Table 1)