Figure 4.
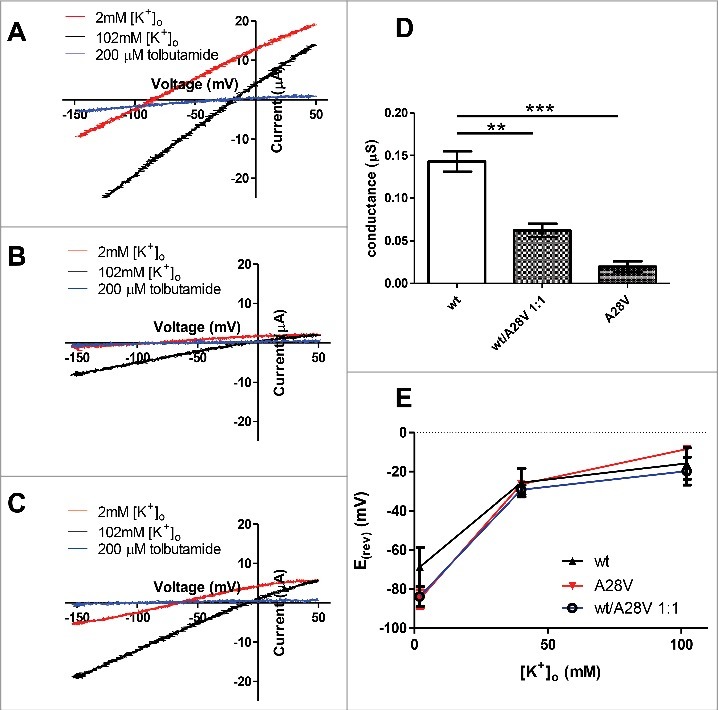
Two-electrode voltage clamp recording of KATP currents in the Xenopus oocytes. (A) A representative wild-type KATP current (red trace) was elicited by a voltage ramp pulse (0.5V/s). As predicted, the KATP current was inhibited by 200 µM KATP channel blocker, tolbutamide (blue trace). Shifting extracellular potassium concentration from 2 mM to 100 mM caused a shift of reversal potential, as predicted by the potassium equilibrium potential. (B) A representative A28V hKir6.2 KATP current (red trace) was elicited by a voltage ramp pulse (0.5V/s). This A28V hKir6.2 KATP current was relatively smaller than the wild-type KATP current, but it could be inhibited by 200 µM tolbutamide (blue trace). The KATP channels containing A28V hKir6.2 were still potassium selective, as the reversal potential followed the potassium equilibrium potential (black trace). (C) A representative recording trace from a Xenopus oocyte injected with an equimolar ratio of wt and A28V hKir6.2 mRNA. KATP current (red trace) was elicited by a voltage ramp pulse (0.5V/s). This wt/A28V KATP current size was in between wild-type and A28V hKir6.2 KATP current, and was inhibited by 200 µM tolbutamide (blue trace). The wt/A28V KATP currents were also potassium selective, as the reversal potential followed the potassium equilibrium potential (black trace). (D) Summary of wt, A28V and wt/A28V KATP currents in the Xenopus oocytes. wt KATP currents (n = 7) were significantly larger than A28V KATP currents (n = 6) and wt/A28V KATP currents (n = 8), as determined by one-way ANOVA with posthoc multiple comparison test, (**p = 0.001, ***p < 0.0005). (E) Summary of the reversal potentials of wt, A28V, and wt/A28V KATP currents. In all three groups, the reversal potentials followed closely with the extracellular potassium concentration, indicating this hKir6.2(A28V) mutant is still potassium selective.
