Figure 5.
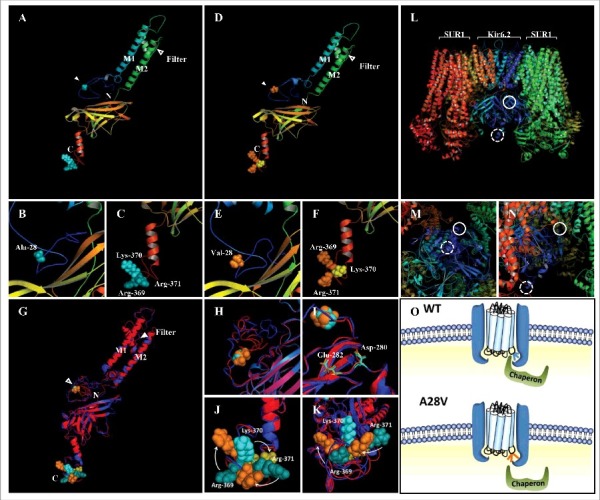
Predicted protein structures of wild-type (A to C) and A28V (D to F) hKir6.2. The first and second transmembrane segments are labeled as M1 and M2, respectively. The selective filter and the position 28 are marked with blank and filled arrowhead, respectively. The N and C represent the N- and C- terminus of the hKir6.2 protein (A and D). (G to K) A superimposed image of the predicted wild-type and A28V hKir6.2. The side chain of position 28 (alanine in wild-type and valine in the mutant) are shown as cyan balls for wild-type and orange balls for the mutant (H and I). Di-acidic motifs of wild-type and A28V hKir6.2 (D280 and E282) are shown as cyan and orange sticks, respectively. (J) The A28V mutation caused a clockwise rearrangement of the RKR motif. (L to N) The fully assembled KATP channel complex (PDB: 5WUA).18 The solid and dashed circles represent the presumptive N-terminal region containing the 28th alanine residue and C- terminal region containing the RKR motif, respectively. (O) A plausible molecular mechanism of the A28V mutation on KATP channel trafficking. In KATP channel formed by the wild-type Kir6.2, the RTR motif is hinged on the neighboring SUR1, and the chaperone may dock onto the KATP channel complex to facilitate the assembled channel complex exiting the ER. In KATP channel formed by the A28V Kir6.2, the C-terminus is distorted and the RKR motif is no longer hidden. The exposed RKR motif may cause a hindrance for the chaperone docking and hence, prevent the forward trafficking of the mutated KATP channel.
