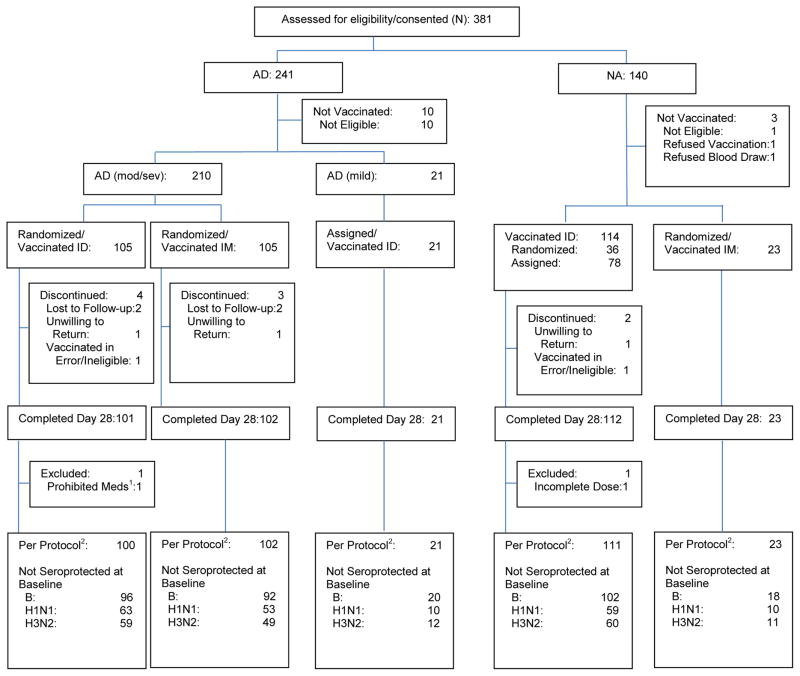
Figure 1. Consort Diagram of Study Participants.
Note: NA=Non-Atopic; AD=Atopic Dermatitis; ID=Intradermal; IM=Intramuscular; Mod/Sev=Moderate/Severe.
1Methotrexate (not allowed during the study)
2The per protocol population includes participants who 1) received a full dose of vaccine, 2) provided serum samples at baseline and day 28, 3) met eligibility criteria, 4) received no prohibited medications, and 5) had no major protocol deviations.