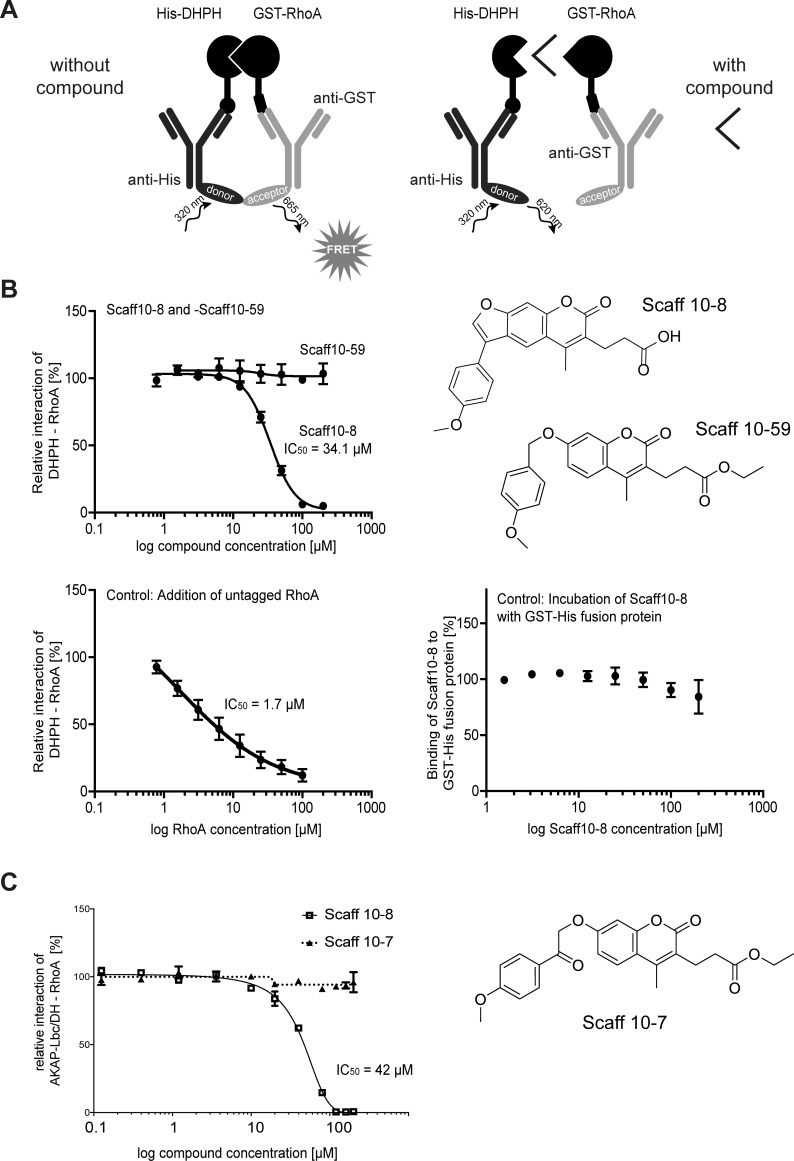
Fig 2. The small molecule Scaff10-8 inhibits the interaction of the DH domain of AKAP-Lbc and RhoA.
(A). Principle of homogenous time-resolved fluorescence (HTRF) assay. Recombinant His-AKAP-Lbc/DHPH and GST-RhoA were generated. The tags are recognized by specific antibodies coupled to fluorescent dyes. When the two proteins interact, donor and acceptor dyes of the antibodies are in close proximity, energy transfer occurs upon excitation at 320 nm and a FRET signal is detected (665 nm). If a compound disrupts the interaction of the DHPH domain and RhoA, the proximity of the fluorescent moieties remains insufficient for a FRET signal to occur. (B) In the HTRF from (A), Scaff10-8 inhibited the interaction of AKAP-Lbc/DHPH and RhoA with an IC50 = 34.1 μM whereas Scaff10-59 did not (example of a negative Scaff10 derivative). As expected, excess untagged Rho decreases the binding between GST-RhoA and His-AKAP-Lbc/DHPH in a dose-dependent manner (lower left panel). Scaff10-8 does not interact with a His-GST fusion protein, excluding any interference of the tags or antibodies used in this assay with Scaff10-8. n = 3–15 independent experiments in duplicate; IC50 values are indicated. (C) The influence of Scaff10-8 on the interaction of RhoA with the recombinant DH domain of AKAP-Lbc in the absence of the PH domain was determined by Alpha screen. Scaff10-7/59 was included as negative controls as they did not interfere with interaction of the DHPH domain and RhoA in the HTRF shown in Fig 2. n = 3–15 independent experiments in duplicate. The IC50 value is indicated.