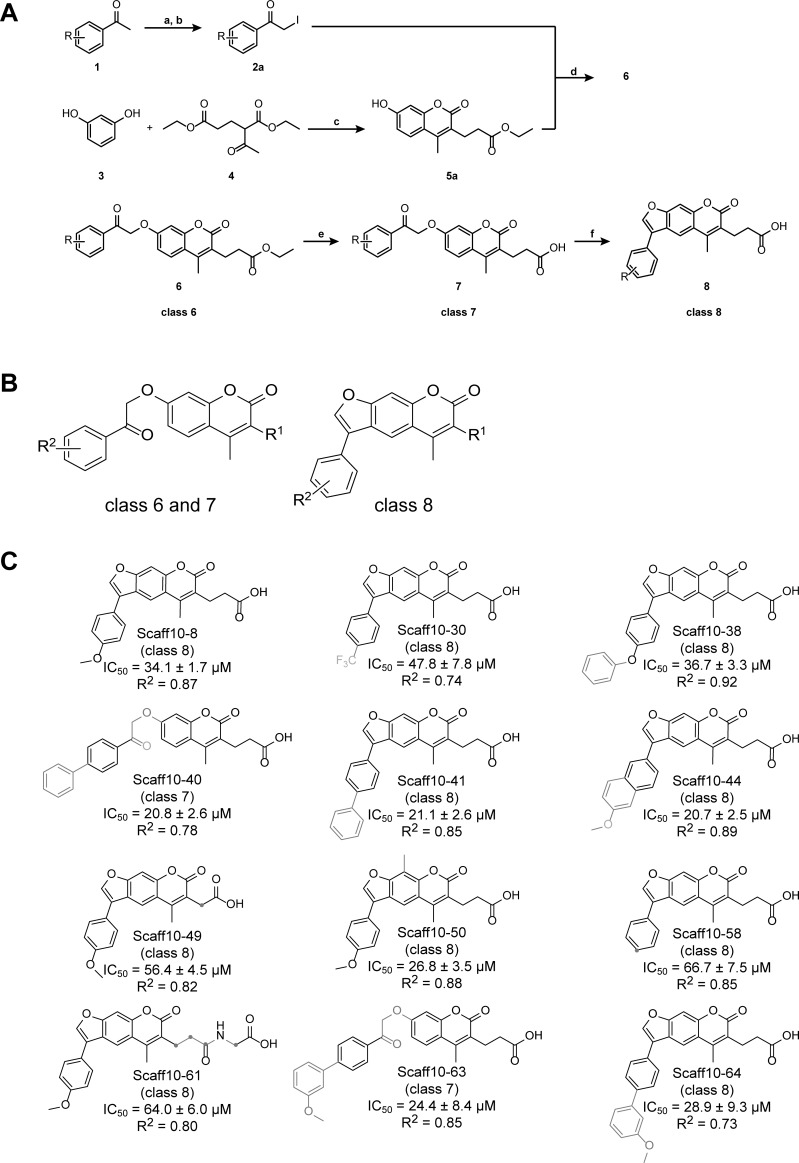
Fig 3. Scaff10 derivatives.
(A) Overview of the synthesis of Scaff10 derivatives. By addition of iodine in methanol (a), acetophenone derivatives (1) reacted with the corresponding α-iodoketones (2a), removal of excessive iodine by addition of Na2SO3-solution (b). Resorcinol (3) and diethyl-2-acetylglutarat (4) were transformed into the 7-hydroxycoumarin derivative ethyl 3-(7-hydroxy-4-methyl-2-oxo-chromen-3-yl)propanoate (5a) in ethanolic HCl (c). In the presence of excess potash, 5 reacted with α-haloketone derivatives (2) in a Williamson ether synthesis at 55°C in acetone (d) to 6. Saponification to 7 was carried out in 1 M NaOH at 55–95°C and from 0.3–16 h (e). Final cyclization of ketones to furocoumarin derivatives (8) was carried out upon further heating in NaOH solution (60–110°C) for various times (0.75–10 h) (f). R is indicated in Table 1 and (C). (B) Compounds of the generalized structures 6–8 from (A) were allocated into classes 6–8, respectively. (C) Structures of Scaff10 derivatives, which inhibit the AKAP-Lbc/DHPH-RhoA interaction in the homogenous time-resolved fluorescence (HTRF) assay depicted in Fig 3A and B. IC50 values (μM ± SEM) were obtained from n = 3–15 independent HTRF experiments carried out in duplicate (S3 Table). Structural differences compared to Scaff10-8 are shown in grey. R2 indicates the coefficient of determination.