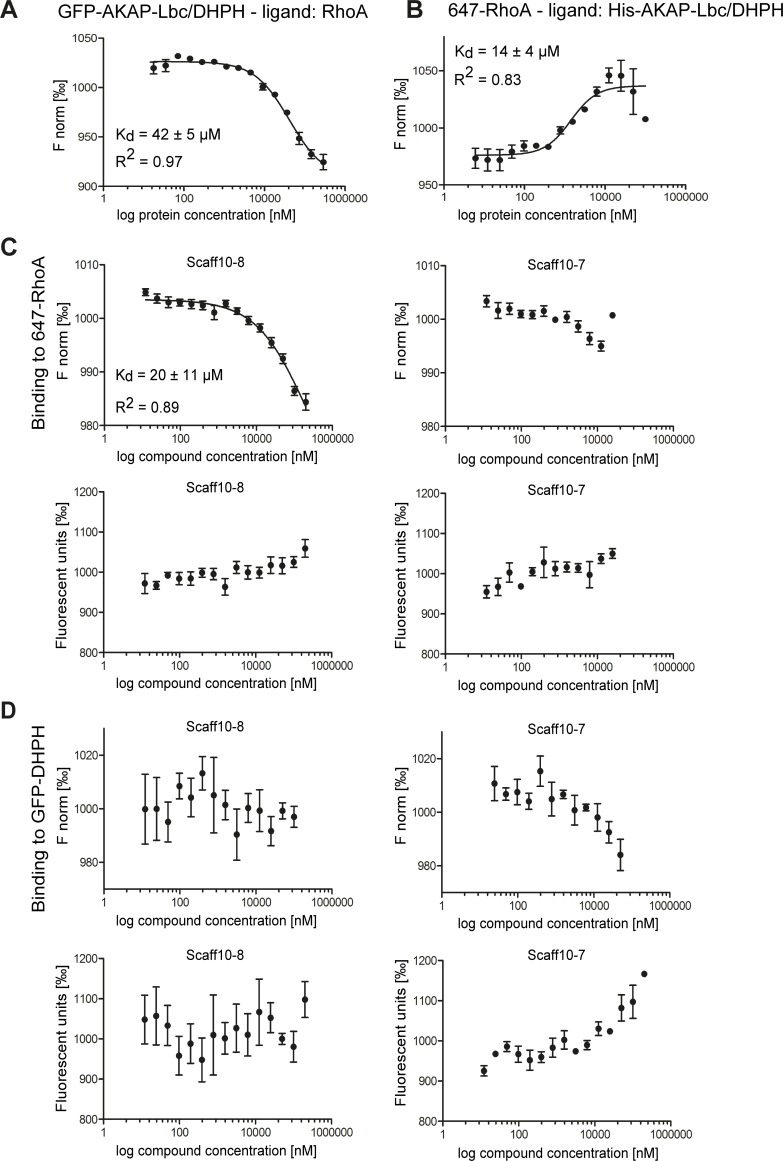
Fig 4. The Scaff10 derivative Scaff10-8 binds to RhoA but not to the DHPH domain of AKAP-Lbc.
(A-D) Microscale thermophoresis (MST) takes advantage of the phenomenon of directed movement of particles in a temperature gradient. Binding events lead to changes in the hydration shell of biomolecules and a relative change of movement of the molecular complex along a temperature gradient. Using such changes, binding affinities can be determined [47]. MST assays were carried out with (A) the recombinant DHPH domain of AKAP-Lbc fused with GFP and RhoA as a ligand and (B) fluorescent 647-RhoA and the His-tagged AKAP-Lbc/DHPH domain as a ligand. (C) MST assays for the analysis of the binding of Scaff10-8 to 647-RhoA. Upper panels: The concentration of fluorescent 647-RhoA remained constant and Scaff10-8 (left) and Scaff10-7 as a negative control (right) were titrated in increasing concentrations. Lower panels: Values of fluorescence corresponding to upper panels. (D) MST assay showing no binding of Scaff10-8 or Scaff10-7 to GFP-DHPH. Upper panels: The concentration of fluorescent GFP-AKAP-Lbc/DHPH remained constant and Scaff10-8 (left) and Scaff10-7 (right) were titrated in increasing concentrations. Lower panels: Values of fluorescence corresponding to upper panels. The KD value for the binding of Scaff10-8 to 647-RhoA is 20 ± 11 μM. F norm = normalized fluorescence (fluorescence steady state/fluorescence initial state) indicated in ‰. n = 3–5. Mean ± SEM.